Cellulose-based Schiff base fluorescent material as well as preparation method and application thereof
A technology based on Schiff bases and fluorescent materials, which is applied in the field of fluorescence detection, can solve problems such as contamination of the system to be tested, fluorescence quenching, and difficulty in processing small fluorescent molecules, and achieve good luminescence performance and mature preparation technology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation method of cellulose-based Schiff base fluorescent material, the reaction formula is:
[0032]
[0033] Specific steps are as follows:
[0034] (1) Preparation of dialdehyde cellulose: carry out alkali treatment to microcrystalline cellulose with 14% sodium hydroxide, get 3.0 g of microcrystalline cellulose after alkali treatment, 150 mL of deionized water, 4.5 g of sodium periodate, adjust The pH value was 2.6, and reacted in the dark at 45°C for 5 hours. After the reaction was completed, 15 mL of ethylene glycol was added to terminate the reaction, and IO was washed away by filtration and distilled water 3 - , Vacuum drying at 45° C. for 24 hours to obtain dialdehyde cellulose.
[0035] (2) Disperse 0.5g dialdehyde cellulose in absolute ethanol, then add 0.01g 4-(4-dimethylaminophenyl)-8,9,9-trimethyl-5,6,7, 8-tetrahydro-5,8-methanoquinazolin-2-amine was dissolved in absolute ethanol and added slowly. Then add 4 drops of acetic acid dropwise, and...
Embodiment 2
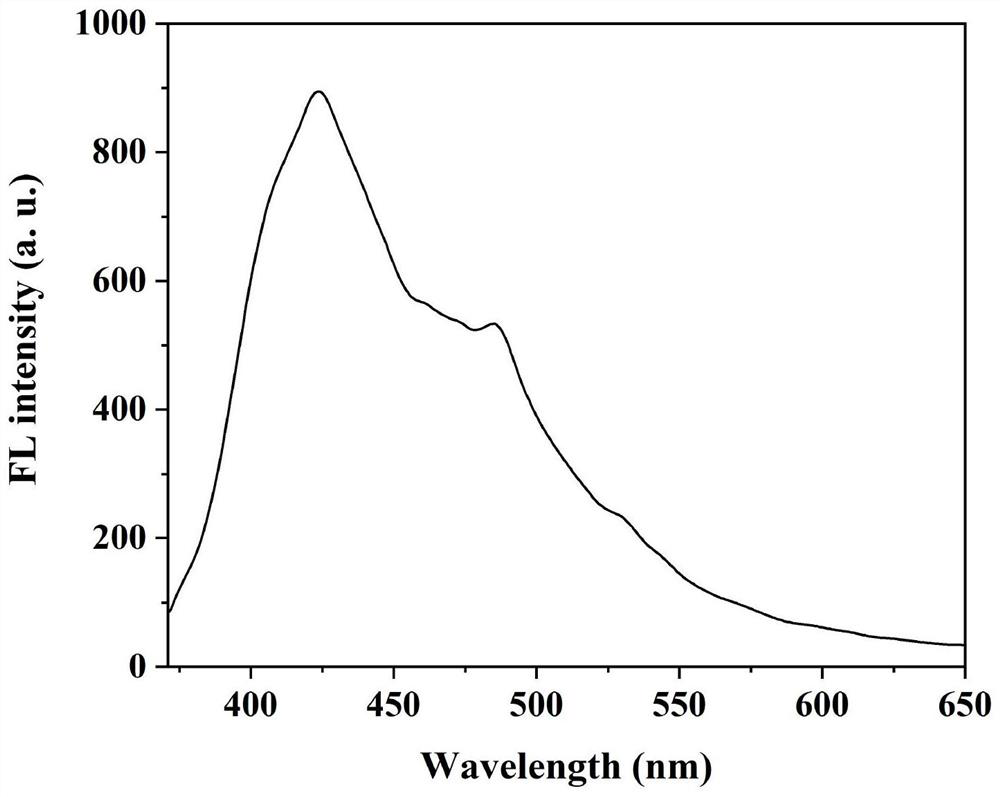
[0038] The cellulose-based Schiff base fluorescent material prepared in Example 1 was pressed into a sheet, and the solid fluorescence emission spectrum of the fluorescent cellulose was measured, as shown in figure 1 shown. The results show that the maximum emission wavelength of solid fluorescence is at 425nm.
Embodiment 3
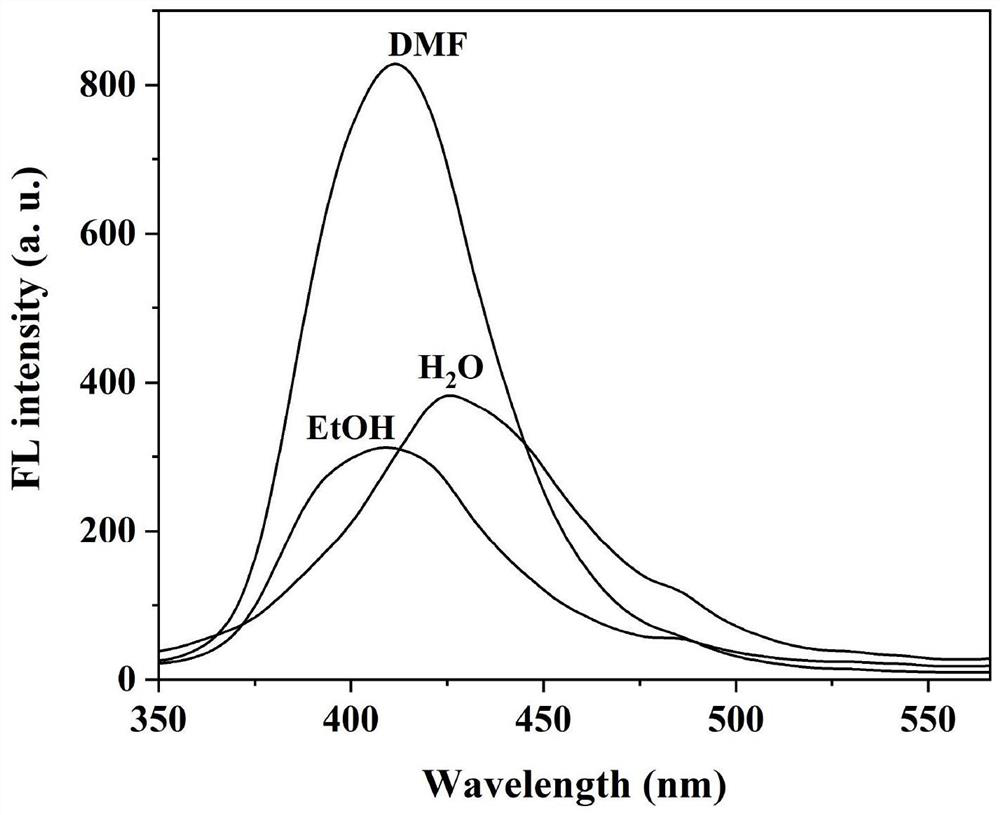
[0040] The cellulose-based Schiff base fluorescent material prepared in Example 1 was prepared in DMF, absolute ethanol and deionized water respectively to prepare a suspension of 0.002 g / 10 mL. Fluorescence emission spectra were measured in DMF, absolute ethanol and deionized water, such as figure 2 shown. The results show that, under the DMF system, the maximum fluorescence emission wavelength of the compound is at 412nm; under the absolute ethanol system, the maximum fluorescence emission wavelength of the compound is at 409nm; under the deionized water system, the maximum fluorescence emission wavelength of the compound is at 426nm place.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com