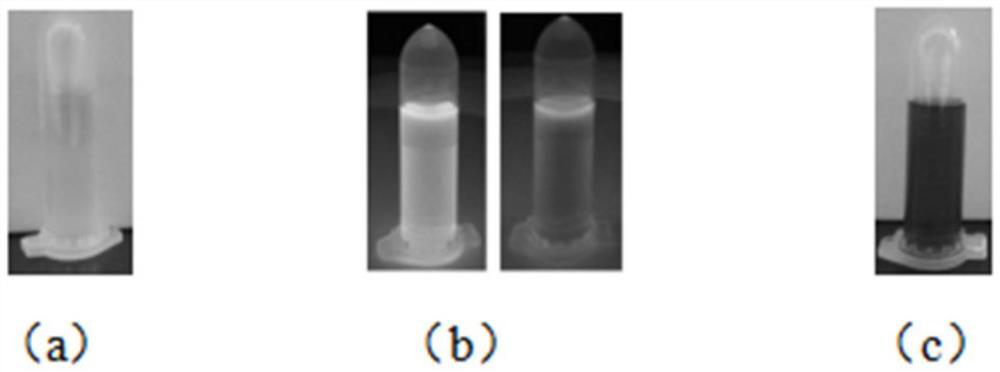
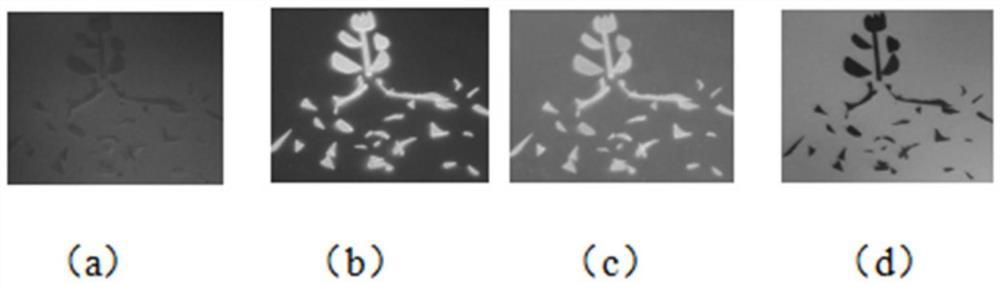
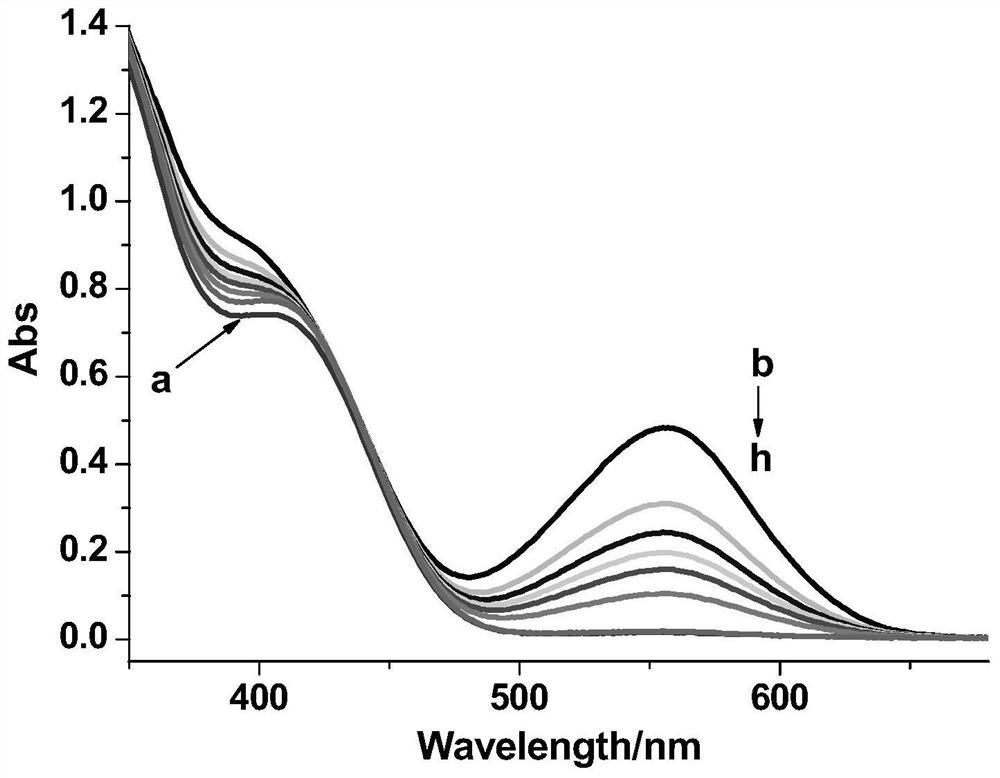
Light-operated dual-channel fluorescent dye and preparation method and application thereof
A fluorescent dye, dual-channel technology, applied in the field of light-controlled dual-channel fluorescent dyes and their preparation, can solve the problems of poor photochemical stability, low fluorescence quantum yield and the like, achieve improved fluorescence intensity, avoid low fluorescence quantum yield, Effect of increasing photochemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The preparation method of the above-mentioned light-controlled dual-channel fluorescent dye comprises the following steps:
[0037] S1, using absolute ethanol as a solvent, 4-bromo-1,8-naphthalene dicarboxylic anhydride and glycine tert-butyrate as raw materials, stirring and reflux temperature, to prepare intermediate 1 through condensation reaction;
[0038] S2, dissolving the intermediate 1 prepared in S1 in ethylene glycol monomethyl ether, adding diethanolamine, stirring, and preparing intermediate 2 through a nucleophilic substitution reaction at reflux temperature;
[0039]S3. Mix the intermediate 2, 1-carboxyethylspiropyran, dicyclohexylcarbodiimide, 4-dimethylaminopyridine and dichloromethane prepared in S2, and carry out esterification under room temperature and dark conditions. Reaction makes intermediate 3;
[0040] S4. The intermediate 3 prepared in S3 is first subjected to a deprotection reaction, and then mixed with N-hydroxysuccinimide, dicyclohexylcarb...
Embodiment 1
[0045] A method for preparing a light-controlled dual-channel fluorescent dye, comprising the following steps:
[0046] (1) Synthesis of intermediate 1
[0047] The amount of 4-bromo-1,8-naphthalene dicarboxylic anhydride and glycine tert-butyrate in absolute ethanol was stirred and refluxed for 4 hours, the consumption of 4-bromo-1,8-naphthalene dicarboxylic anhydride and absolute ethanol The ratio is 1mmol: 30mL, after cooling to room temperature, pour it into deionized water, add ethyl acetate for extraction, dry the organic layer with anhydrous magnesium sulfate, filter out the desiccant, add activated carbon for decolorization, evaporate most of the solvent, and cool Set, and filtered to obtain a light yellow solid, which is intermediate 1.
[0048] 1 H NMR [CDC1 3 ,300MHz]δ=1.49(s,9H),4.83(s,2H),7.85(t,1H),7.04(d,1H),8.42(d,1H),8.58(d,1H),8.66(d ,1H).
[0049] (2) Synthesis of intermediate 2
[0050] Dissolve 1.65g (4mmol) of intermediate 1 in 50mL of ethylene glyc...
Embodiment 2
[0062] A method for preparing a light-controlled dual-channel fluorescent dye, comprising the following steps:
[0063] Step is with embodiment 1, and difference is:
[0064] In step (1), the molar ratio of 4-bromo-1,8-naphthalene dicarboxylic anhydride and glycine tert-butyrate is 1:1.5, the amount of 4-bromo-1,8-naphthalene dicarboxylic anhydride and absolute ethanol The ratio is 1mol: 20L, and the reaction time is 3.5h;
[0065] In step (2), the dosage ratio of intermediate 1 and diethanolamine is 1mol:1L, the volume ratio of ethylene glycol monomethyl ether and diethanolamine is 40:3, and the reaction time is 9.5h;
[0066] In step (3), the ratio of intermediate 2: 1-carboxyethylspiropyran: dicyclohexylcarbodiimide: dichloromethane is 1mmol: 2.2mmol: 2.4mmol: 75mL; intermediate 2: 4 -The molar ratio of dimethylaminopyridine is 10:1, and the reaction time is 11.5h;
[0067] In step (4), the mol ratio of intermediate 3: N-hydroxysuccinimide: dicyclohexylcarbodiimide is 1:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com