L-proline 4-hydroxylase and application thereof
A technology of proline and hydroxylase, applied in the direction of application, oxidoreductase, enzyme, etc., can solve the problems of limited industrial application, low catalytic activity, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Homologous Modeling and Evaluation
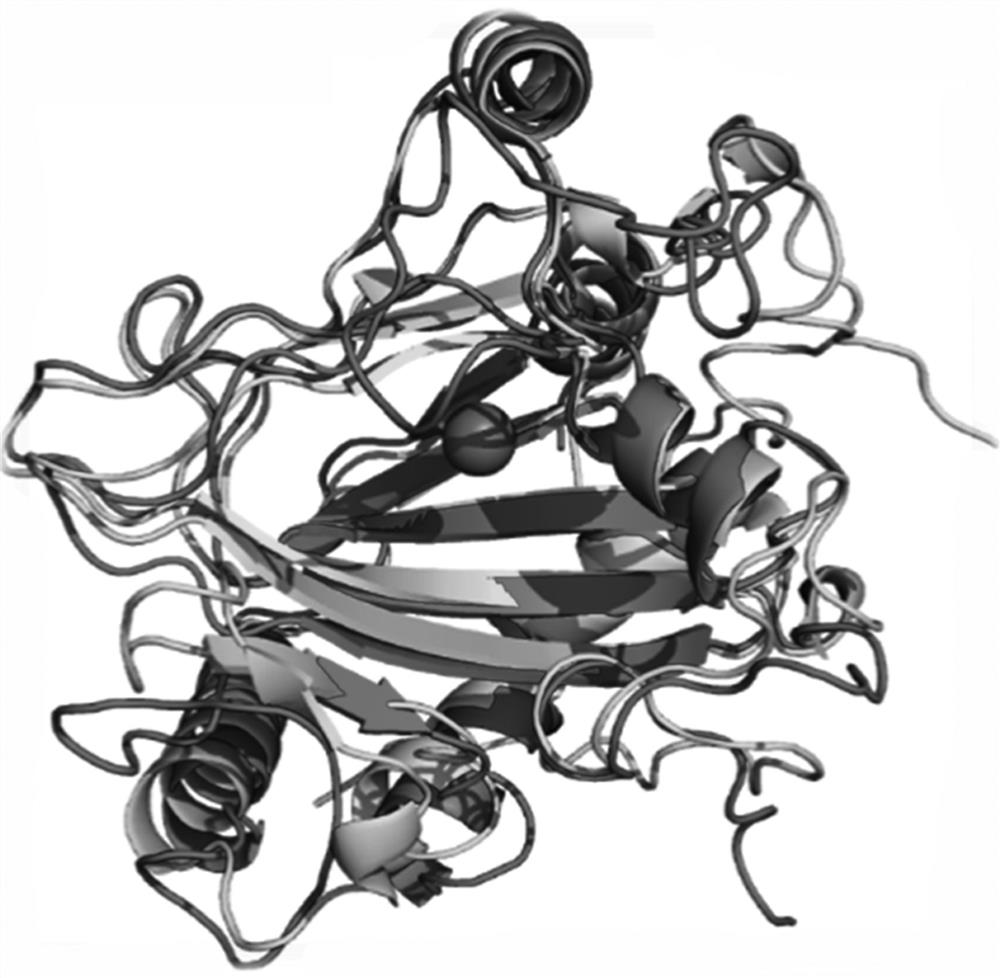
[0041] Using the SWISS-MODEL homology modeling server (https: / / www.swissmodel.expasy.org / ) to perform homology modeling on proline hydroxylase (BP4H) from Bacillus cereus HBU-AI, specifically The steps of the method are as follows: input the target sequence, search for a suitable template, and then select a suitable template for homology modeling. The experimental results of P4H structure research are few, and the ectoine hydroxylase (PDB: 4q5o) with high homology to the target sequence is selected as a template, and the automatic mode (Automated Mode) is used for modeling. The Ramachandran plot of the Procheck program and the verify3D model structure were used to score, and the compatibility of the three-dimensional structure with its primary sequence was evaluated.
[0042] The results show that: inputting the BP4H protein sequence to the SWISS-MODEL homology modeling server for sequence comparison, it is found that the ...
Embodiment 2
[0043] Example 2 Sequence Conservation Analysis and Molecular Docking
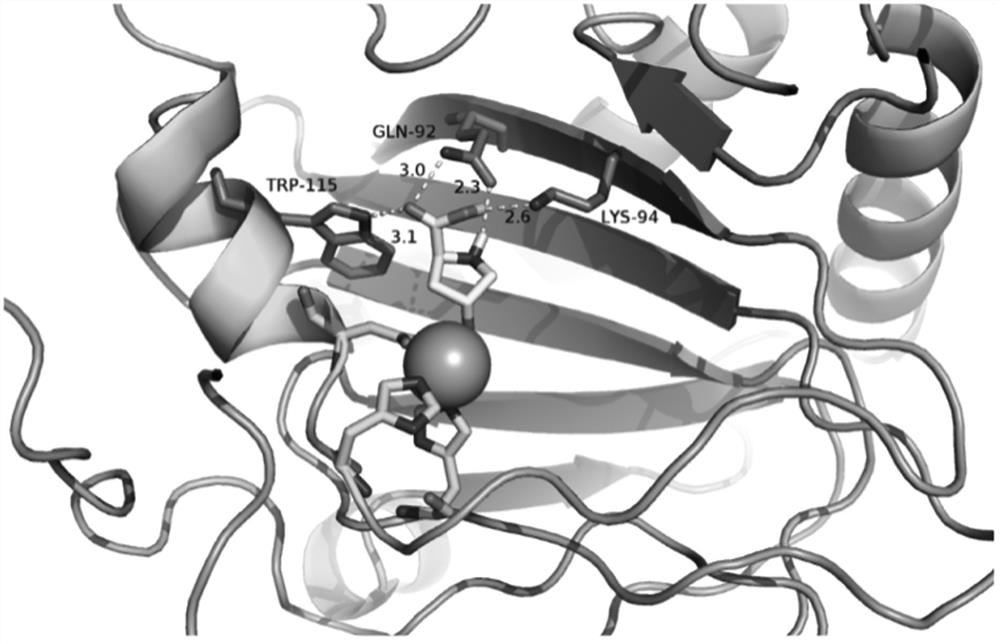
[0044] Sequence homology comparison search found that proline hydroxylase gene and ectoine hydroxylase gene ( ect D) have high homology and belong to Fe 2+ Binds Fe to the α-ketoglutarate-dependent dioxygenase superfamily 2+ The conserved 2-His-1-carboxylate facet triplet (see image 3 ) and the conserved site RXS motifs that bind α-ketoglutarate (see Figure 4 ). The enzymatic functions of the non-ferrorubin- and α-ketoglutarate-dependent dioxygenase families are largely dependent on highly reactive iron ions. Proteins Superimposing reveals that in the active center of proline hydroxylase BP4H, the functional groups (2 imidazole groups and 1 carboxyl group) of amino acid residues His109, Asp111 and His215 interact with Fe 2+ Combine to form the so-called 2-His-1-carboxylate triad. In all non-ferrirubin- and α-ketoglutarate-dependent enzymes of the dioxygenase family, the co-substrate α-ketoglutarat...
Embodiment 3
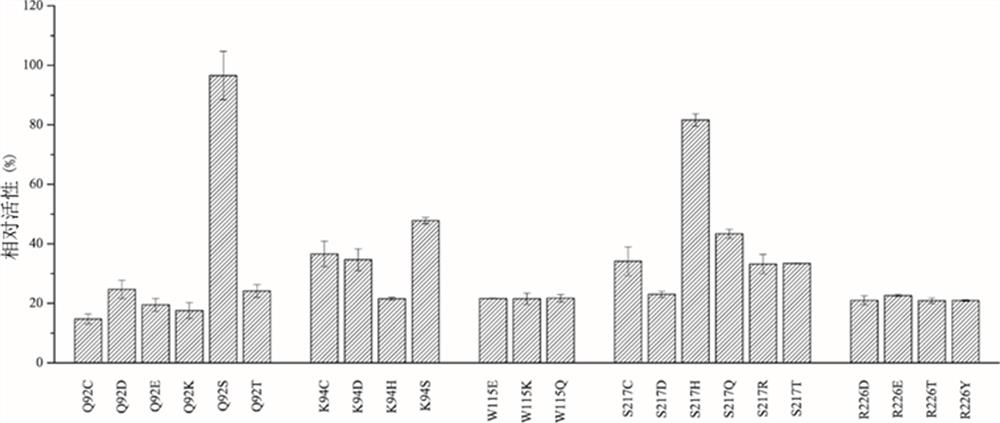
[0046] Example 3 BP4H enzyme site-directed half-saturation mutation
[0047] To introduce a single-base site-directed mutation into a plasmid, it is only necessary to design a pair of primers to amplify the plasmid by reverse PCR. The sequences of the designed primers are shown in Table 1 below. The degenerate primers encoding all polar amino acids designed at a key amino acid site were used to amplify the plasmid containing the BP4H gene by PCR, and the amplified product was detected by agarose gel electrophoresis. Finally, we successfully obtained the BP4H gene containing the mutation plasmid with clear bands.
[0048] Table 1 Primer sequences
[0049] .
[0050] Note: n stands for four nucleotides; v stands for A / C / G; k stands for G / T, n, v, k encode almost all polar amino acids, suitable for us to verify the substitution of these five amino acid positions sex.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com