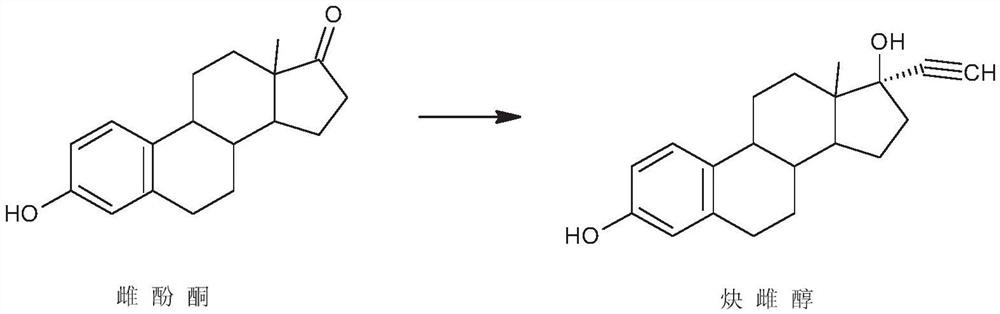
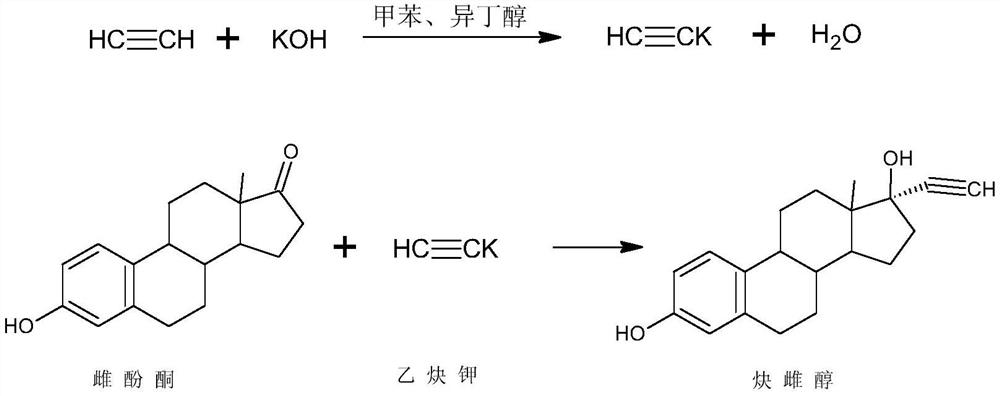
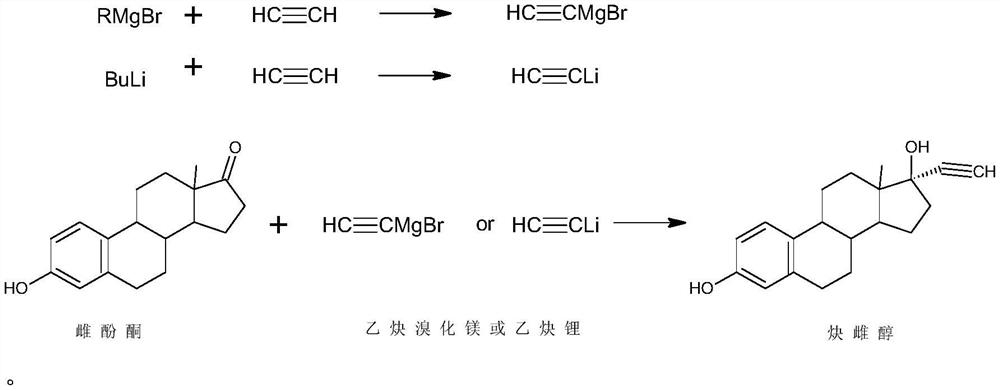
Preparation method of ethinylestradiol
A technology for ethinyl estradiol and estrone, which is applied in the field of drug preparation, can solve the problems of low production efficiency, difficult process control, long reaction time, etc., and achieves the effects of low reaction device requirements, improved reaction efficiency, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 ethinyl estradiol
[0024] Dissolve 50g of estrone in 250ml of tetrahydrofuran, add 50g of potassium tert-butoxide, control 5°C, pass through acetylene, stir the reaction, after the reaction, neutralize with 5% hydrochloric acid aqueous solution, concentrate, add water for water analysis, filter to obtain the crude product , the crude product was refined with ethanol and dried to obtain 46 g of ethinyl estradiol, the mass yield of the product was 92%, the melting point of the product was 182.0-183.5° C., and the HPLC content was 99.5%.
Embodiment 2
[0025] The preparation of embodiment 2 ethinyl estradiol
[0026] Dissolve 50g of estrone in 100ml of dimethyl sulfoxide and 800ml of toluene, add 70g of potassium isobutoxide, control the temperature at 30°C, pass through acetylene, and stir the reaction. After the reaction, neutralize with 10% sulfuric acid aqueous solution, concentrate, Water was added for water analysis and filtered to obtain a crude product, which was refined with methanol and dried to obtain 47 g of ethinyl estradiol. The mass yield of the obtained product was 94%, the melting point of the product was 182.5-183.9° C., and the HPLC content was 99.6%.
Embodiment 3
[0027] The preparation of embodiment 3 ethinyl estradiol
[0028] Dissolve 50g of estrone in 1500ml of acetone, add 250g of sodium ethoxide, control the temperature at -20°C, pass through acetylene, stir the reaction, after the reaction, neutralize with 35% acetic acid aqueous solution, concentrate, add water for water analysis, filter to obtain the crude product , the crude product was refined with acetone and dried to obtain 45.5 g of ethinyl estradiol, the mass yield of the product was 91%, the melting point of the product was 182.1-183.2°C, and the HPLC content was 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com