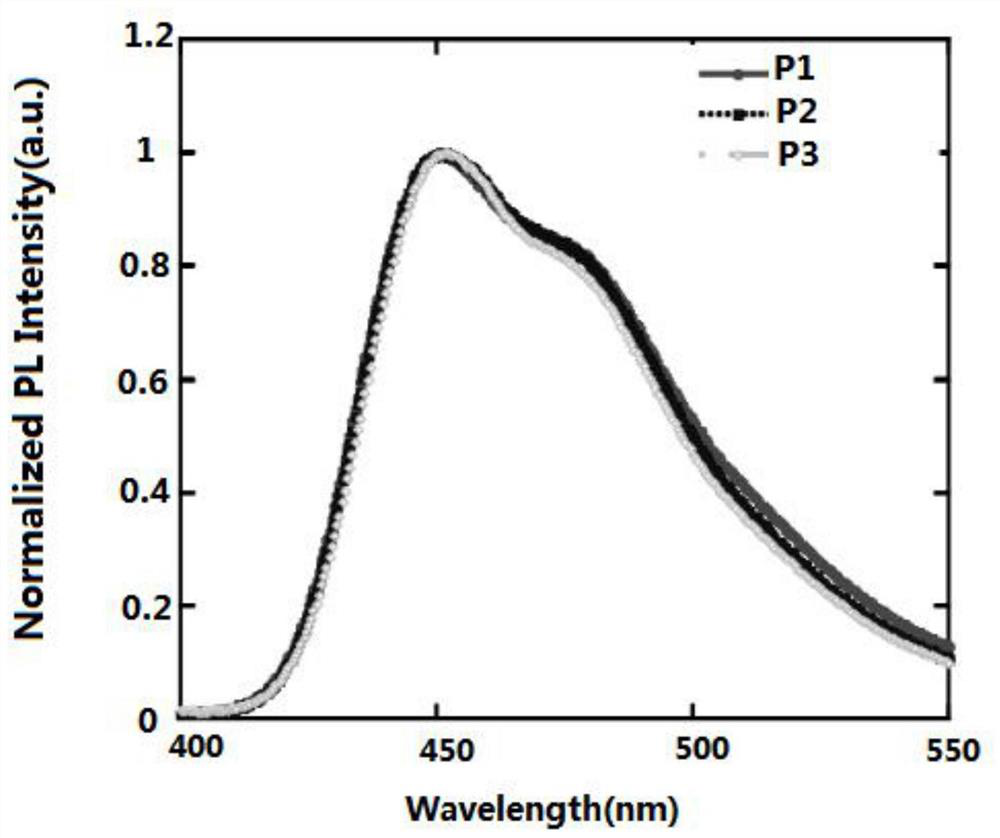
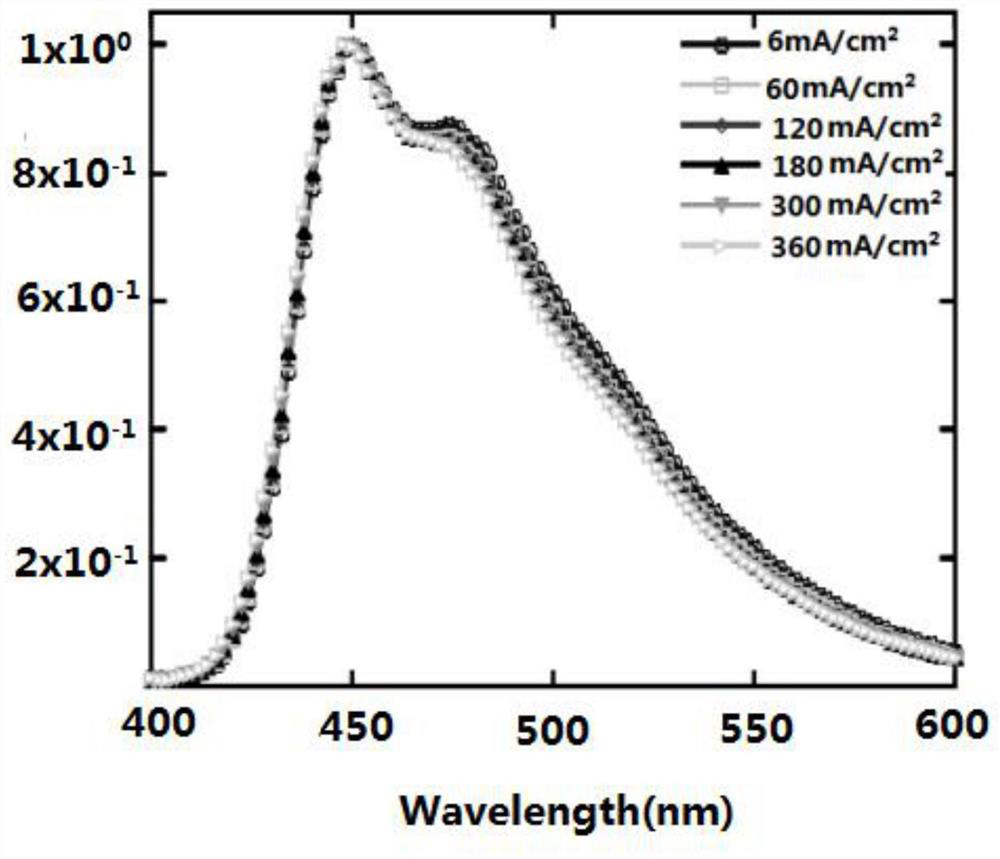
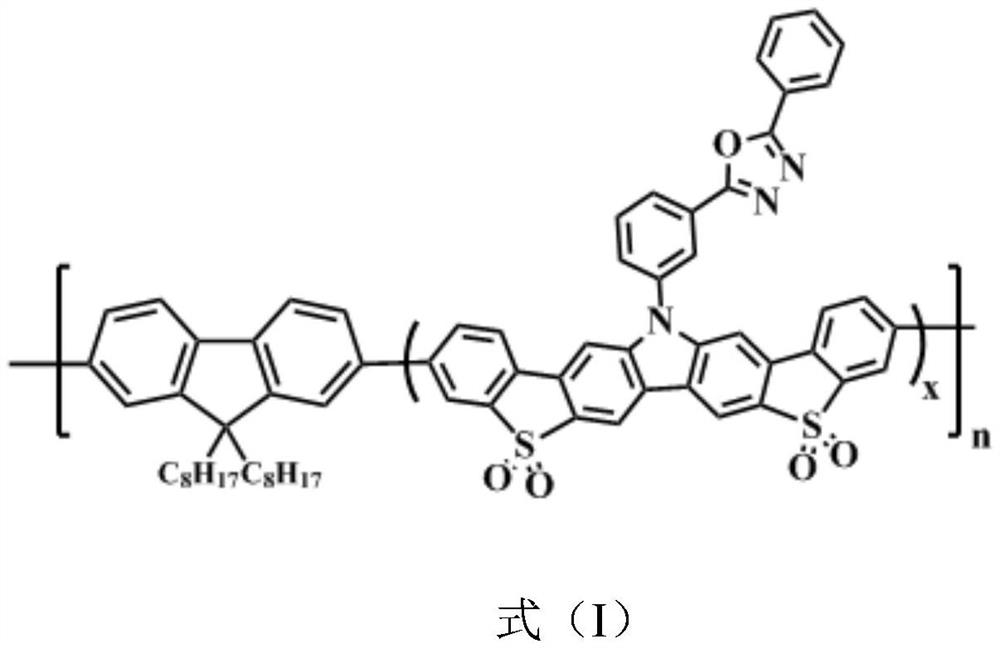
Blue light organic electroluminescent material and preparation method thereof
An electroluminescence, electromechanical technology, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of poor electroluminescence performance and difficult preparation of blue polymer, and achieve good device effect and high spectral stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Dissolve 4,4'-dibromobiphenyl (15.6g, 50mmol) in 150mL of acetic acid, stir under ice bath in the dark, take 30ml of fuming nitric acid and dilute with 50ml of acetic acid, add to the constant pressure dropping funnel, under dark conditions Slowly add dropwise to the reaction solution. After the addition is completed, the reaction temperature is slowly increased to 85°C and refluxed for 2 hours. The change of the reaction solution is observed. The reaction is stopped when the reaction solution turns from turbidity to transparent, and it is naturally cooled to room temperature. The reaction solution was slowly poured into ice water for quenching, the mixed solution was suction filtered, the filter residue was washed with water, saturated sodium bisulfite aqueous solution, water, ice ethanol, and then the filter residue was dried to obtain 4,4'-dibromo-2-nitrate The reaction equation of biphenyl (12.7g, yield 63%) is as follows:
[0018]
Embodiment 2
[0020] 4,4'-Dibromo-2-nitrobiphenyl (14.28g, 40mmol) and triphenylphosphine (26.2g, 100mmol) were dissolved in 250ml of N,N-dimethylacetamide (DMA), argon Heat and stir overnight in an atmosphere. The reaction was stopped and cooled to room temperature, extracted with dichloromethane and washed with water 3-5 times, purified by column chromatography, 200-300 mesh silica gel was used as the stationary phase, and petroleum ether / dichloromethane (2:1) was used as the washing The agent was removed and subjected to silica gel column chromatography, and the crude product was recrystallized from ethanol to obtain 2,7-dibromocarbazole (9.7g, yield 74%). The reaction equation is as follows:
[0021]
Embodiment 3
[0023] Dissolve 2,7-dibromocarbazole (6.5g, 20mmol) in 50ml of N,N-dimethylformamide (DMF), add potassium hydroxide (2.7g, 48mmol), stir at room temperature for 30min, then add 2-(3-Bromophenyl)-5-phenyl-1,3,4-oxadiazole (12.94g, 43mmol), after the addition is complete, continue stirring at 85°C for 10h. After the reaction was completed, the reaction solution was poured into water, and a light yellow solid precipitated. The solid is filtered under reduced pressure and rinsed with distilled water several times, and then drained under reduced pressure to obtain 2-(3-(2,7-dibromo 9H-carbazol-9-yl)phenyl)-5-phenyl -1,3,4-oxadiazole (9.8g, yield 90%), the chemical equation of the reaction is:
[0024]
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com