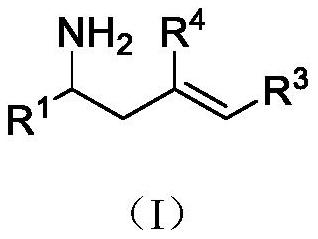
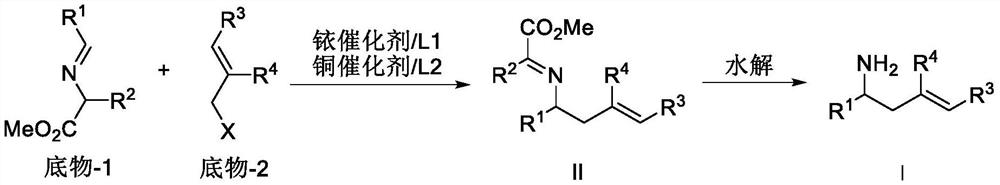
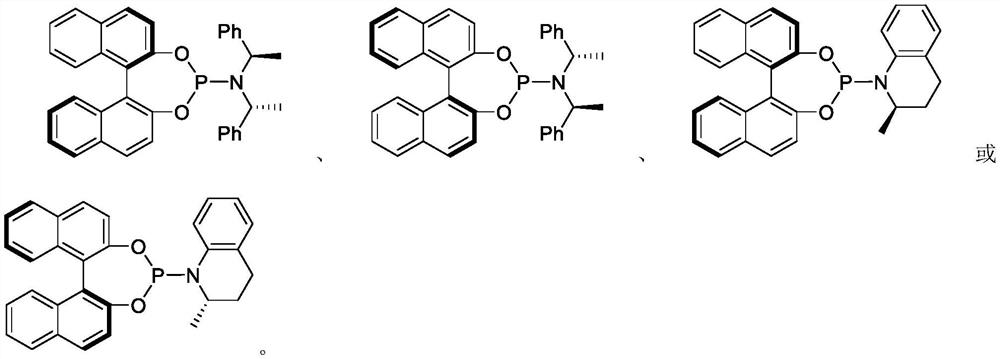
Copper/iridium synergistically catalyzed asymmetric allylation/2-aza-cope rearrangement and its application
A technology of allylation and synergistic catalysis, which is applied in the field of chemical medicine to achieve the effect of high yield, great value and prospect of drug synthesis, and good enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] preparation
[0056] Add 0.003mmol [Ir(COD)Cl] to a 25mL reaction tube 2 , 0.006 mmol (S, S, S)-L1, 0.5 mL of deoxygenated THF and 0.5 mL of deoxygenated n-propylamine, react at 50° C. for 30 minutes and then evaporate the solvent under reduced pressure to obtain an iridium complex. In another 25mL reaction tube, add 0.01mmol Cu(CH 3 EN) 4 BF 4 With 0.011 mmol DPEphos, under nitrogen protection, 1 mL of dichloromethane was added, and the mixture was stirred at room temperature for half an hour. Then at 25°C, 0.30 mmol of methyl 2-(p-chlorobenzylideneamino)isovalerate, 0.20 mmol of methyl cinnamyl carbonate, 0.3 mmol of cesium carbonate and iridium complex were added in sequence, and after stirring for 24 h, Add 0.5mL 2N hydrochloric acid for 0.5h, add 1.0mL 2NNaoH aqueous solution and 0.4mmol Boc 2 O was reacted for 3h, the solvent was evaporated, and the product was subjected to silica gel column chromatography (petroleum ether / ethyl acetate 10:1) to obtain a wh...
Embodiment 2
[0058] preparation
[0059] Add 0.003mmol [Ir(COD)Cl] to a 25mL reaction tube 2 , 0.006 mmol (S, S, S)-L1, 0.5 mL of deoxygenated THF and 0.5 mL of deoxygenated n-propylamine, react at 50° C. for 30 minutes and then evaporate the solvent under reduced pressure to obtain an iridium complex. In another 25mL reaction tube, add 0.01mmol Cu(CH 3 EN) 4 BF 4 With 0.011 mmol DPEphos, under nitrogen protection, 1 mL of dichloromethane was added, and the mixture was stirred at room temperature for half an hour. Then at 25°C, 0.30 mmol of methyl 2-(p-chlorobenzylideneamino)isovalerate, 0.20 mmol of methyl 3-p-tolylallyl carbonate, 0.3 mmol of cesium carbonate and iridium complex were added in sequence After stirring for 24 h, 0.5 mL of 2N hydrochloric acid was added to react for 0.5 h, and 1.0 mL of 2N aqueous NaOH solution and 0.4 mmol Boc were added. 2 O was reacted for 3h, the solvent was evaporated, the product was subjected to silica gel column chromatography (petroleum ether...
Embodiment 3
[0061] preparation
[0062] Add 0.003mmol [Ir(COD)Cl] to a 25mL reaction tube 2 , 0.006 mmol (S, S, S)-L1, 0.5 mL of deoxygenated THF and 0.5 mL of deoxygenated n-propylamine, react at 50° C. for 30 minutes and then evaporate the solvent under reduced pressure to obtain an iridium complex. In another 25mL reaction tube, add 0.01mmol Cu(CH 3 EN) 4 BF 4 With 0.011 mmol DPEphos, under nitrogen protection, 1 mL of dichloromethane was added, and the mixture was stirred at room temperature for half an hour. Then at 25°C, 0.30 mmol 2-(p-chlorobenzylideneamino)isovalerate methyl ester, 0.20 mmol 3-m-tolylallyl methyl carbonate, 0.3 mmol cesium carbonate and iridium complex were added in sequence , after stirring for 24h, add 0.5mL 2N hydrochloric acid for 0.5h, add 1.0mL 2N NaoH aqueous solution and 0.4mmol Boc 2 O was reacted for 3h, the solvent was evaporated, and the product was subjected to silica gel column chromatography (petroleum ether / ethyl acetate 10:1) to obtain a wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com