Preparation method of high-selectivity hypochlorous acid fluorescent probe
A fluorescent probe and high-selectivity technology, applied in the field of fluorescent probes, can solve the problems of insufficient response, high background fluorescence, poor selectivity of hypochlorous acid, etc., and achieve the elimination of background fluorescence interference, sensitive detection effect, and good practical application. potential effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
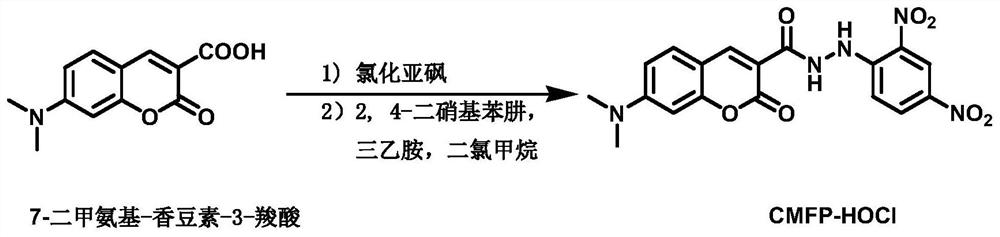
[0032] Synthesis of fluorescent probes;
[0033] Synthetic route such as figure 1 .
[0034] Synthesis of 7-dimethylaminocoumarin-3-acyl chloride: In a 100ml round bottom flask, add 7-dimethylaminocoumarin-3-carboxylic acid (1.2g, 5.15mmol), dropwise add 5ml of Water Thionyl Chloride. N 2 Stir at room temperature under protection for 4h. After suction filtration, the filter cake was washed with cold anhydrous ether to obtain a yellow solid compound 7-dimethylaminocoumarin-3-acyl chloride (0.75 g, yield 57.8%). It was directly used in the next reaction without purification.
[0035] Synthesis of fluorescent probe CMFP-HOCl: In a 100-ml round-bottomed flask, the compound 7-dimethylaminocoumarin-3-acid chloride (0.3 g, 1.2 mmol) was completely dissolved in 10 mL of anhydrous CH 2 Cl 22,4-Dinitrophenylhydrazine (0.15 g, 0.75 mmol) was added thereto, and then triethylamine (2 mL, 14.4 mmol) was added dropwise, and stirred at room temperature for 24 h. After the reaction sto...
Embodiment 2
[0038] Preparation of fluorescent probe and hypochlorous acid solution;
[0039] 1. Weigh an appropriate amount of fluorescent probe CMFP-HOCl and dissolve it in chromatographically pure N, N-dimethylformamide (DMF) to prepare a 1.0 mM fluorescent probe molecular stock solution;
[0040] 2. Weigh an appropriate amount of sodium hypochlorite and dilute it with distilled water to obtain a 10mM hypochlorous acid solution and dilute it step by step to obtain a 10-0.1mM hypochlorous acid solution;
[0041] 3. Weigh an appropriate amount of KH 2 PO 4 、Na 2 HPO 4 , NaCl, and KCl were fixed to volume with distilled water to obtain a phosphate buffered saline solution (PBS) with a concentration of 0.01M and pH=7.4;
[0042] 4. Use acetonitrile and the PBS obtained in step 3 to mix according to the volume ratio of acetonitrile:PBS=4:6, add a certain volume of the fluorescent probe stock solution in step 1 and the hypochlorous acid solution in step 2, and the obtained mixed The conc...
Embodiment 3
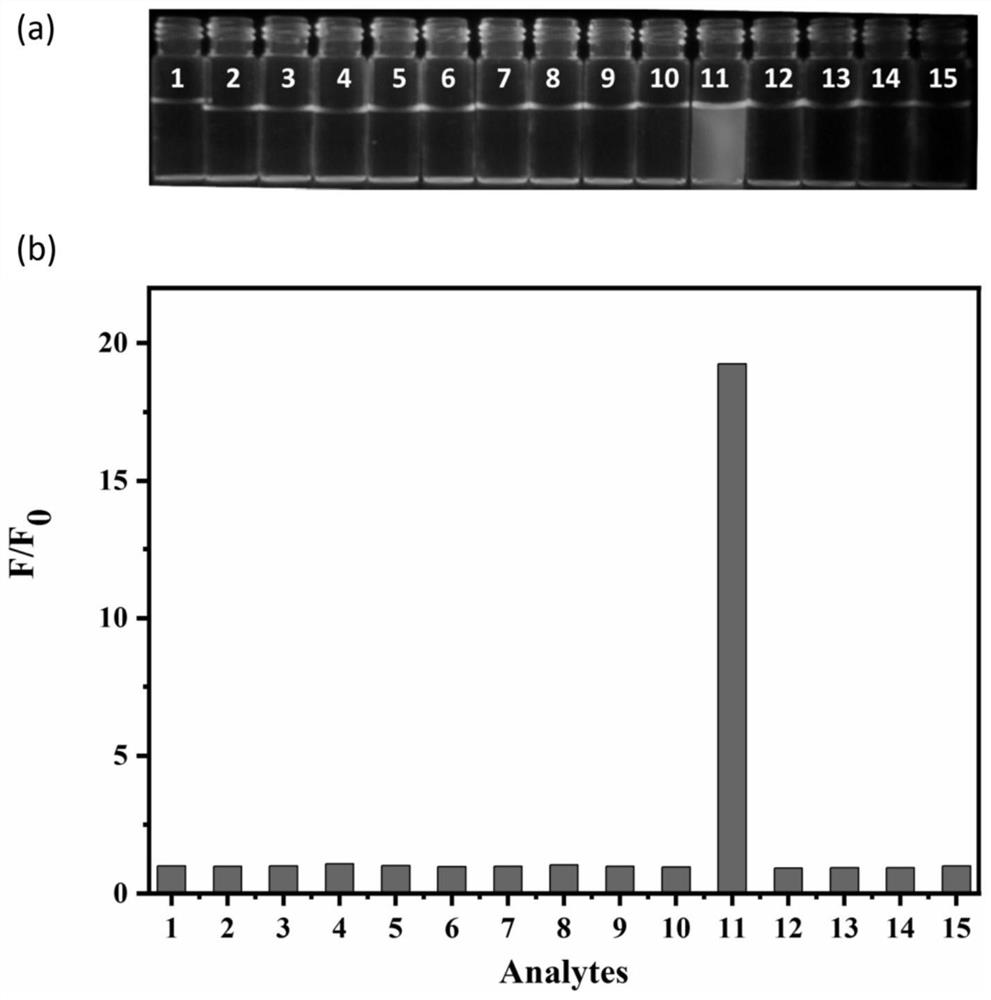
[0044] Fluorescent spectrometry of fluorescent probes in response to HOCl;
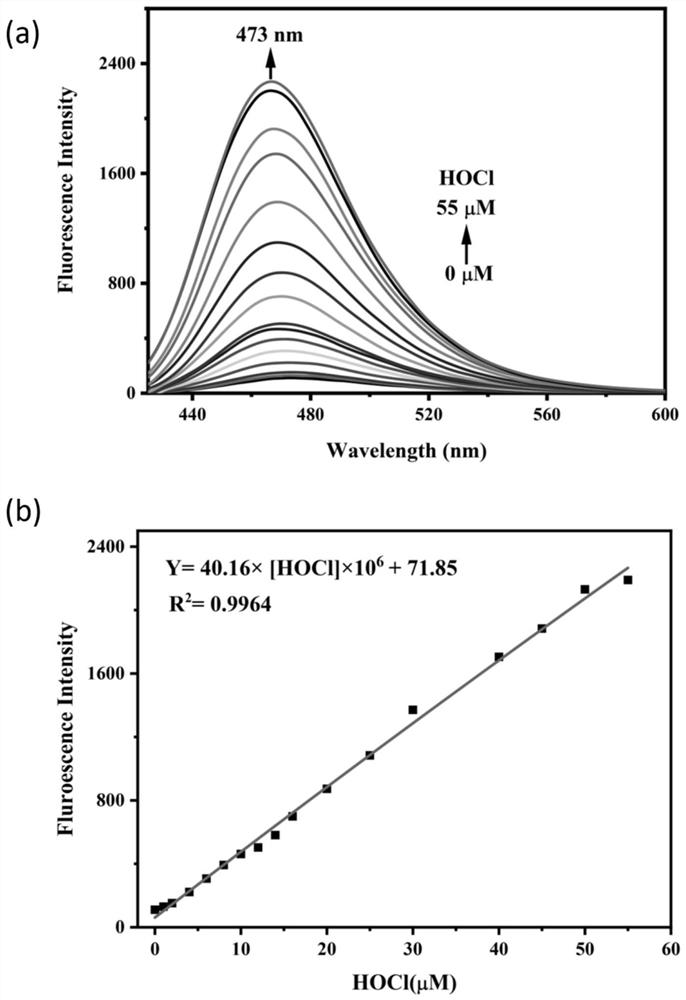
[0045] figure 2 (a) is the fluorescence emission spectrum of the fluorescent probe CMFP-HOCl (10 μM) in response to 0-55 μM hypochlorous acid. The excitation spectrum used in the experiment is 400nm, and the emission wavelength range is 420-600nm. Both the excitation and emission slit widths are 10 nm, and the fluorescence measurement instrument used is a Thermo Fisher Lumina fluorescence spectrophotometer. like figure 2 As shown, the fluorescent probe CMFP-HOCl itself only emits weak fluorescence. After adding hypochlorous acid, the solution emits obvious fluorescence emission at 473nm, and the fluorescence intensity increases with the increase of hypochlorous acid concentration. This is because hypochlorous acid can specifically cut off the hydrazide bond in the fluorescent probe molecule, releasing the fluorescence of the fluorescent group 7-dimethylaminocoumarin-3-carboxylic acid. figure 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com