Method for preparing darunavir intermediate through biological catalysis
A technology of darunavir and biocatalysis, which is applied in the field of biocatalytic preparation of darunavir intermediates, can solve the problems of low enzyme activity and low catalytic reaction efficiency, and achieve high alcohol dehydrogenase activity and high number of coenzyme cycles , The effect of low discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Through the method of whole gene synthesis, the secondary structure and codon bias of the gene are adjusted to achieve high expression in Escherichia coli. Use Primer Premier (http: / / primer3.ut.ee / ) and OPTIMIZER (http: / / genomes.urv.es / OPTIMIZER / ) to design, and ensure that the Tm difference is controlled within 3°C, and the primer length is controlled within 60base , to get splicing primers.
[0022] The above primers were synthesized, and the obtained primers were dissolved in double-distilled water, and then added to the following reaction system, so that the final concentration of each primer was 30 nM, and the final concentration of the first and last primers was 0.6 μM.
[0023] 2mM dNTP mix (2mM each dNTP) 5μl 10×Pfu buffer 5μl Pfu DNA polymerase (10U / μl) 0.5μl wxya 2 o
[0024] The prepared PCR reaction system was placed in the Bioer XP cycler gene amplification instrument, and the amplification was carried out according to the f...
Embodiment 2
[0025] Synthesis of embodiment 2 control protein CKSm gene sequence
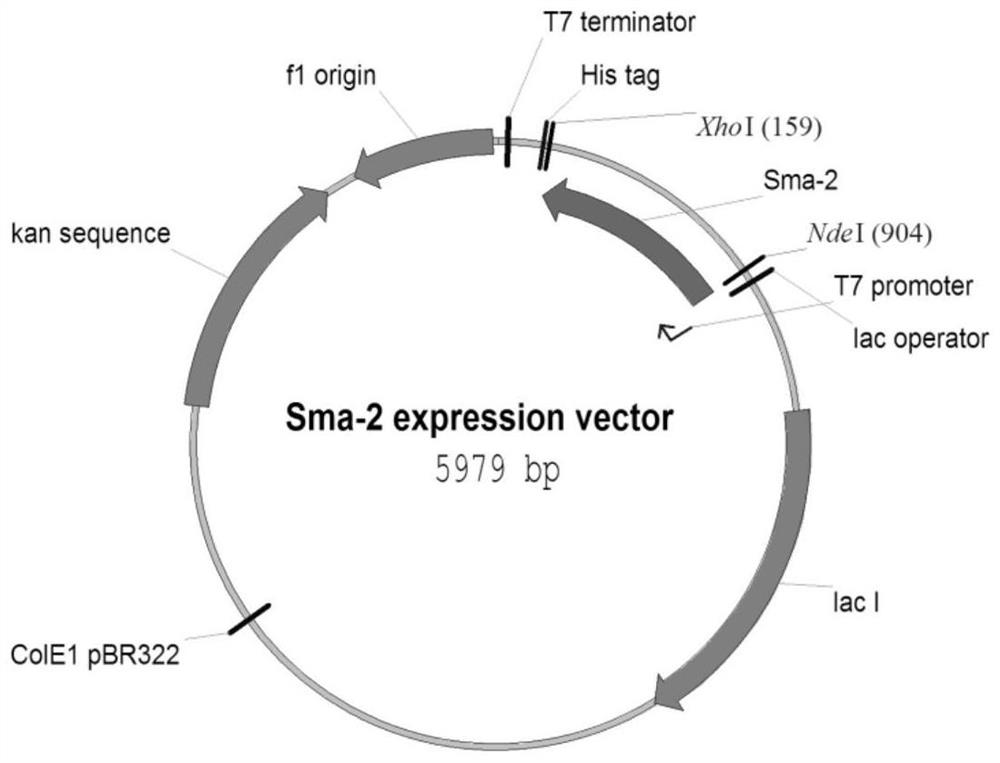
[0026] According to the sequence shown in SEQ ID NO.7, Nanjing GenScript Biology was entrusted to carry out the whole gene synthesis of the coding sequence of the protein, and cloned it into pET30a to obtain the control protein expression plasmid NYK-CKSm. Its corresponding amino acid sequence is SEQID NO.8.
Embodiment 3
[0027] Embodiment 3 shake flask expression test
[0028] Pick a single colony of Escherichia coli containing the expression vector and inoculate it in 10ml of high-pressure sterilized medium: tryptone 10g / L, yeast extract 5g / L, disodium hydrogen phosphate 3.55g / L, potassium dihydrogen phosphate 3.4g / L, Ammonium Chloride 2.68g / L, Sodium Sulfate 0.71g / L, Magnesium Sulfate Heptahydrate 0.493g / L, Ferric Chloride Hexahydrate 0.027g / L, Glycerin 5g / L, Glucose 0.8g / L, Add Calcium Namycin to 50mg / L. Cultivate overnight at 30°C, 250rpm. The next day, take a 1L Erlenmeyer flask and insert it into 100ml of high-pressure sterilized medium at an inoculation ratio of 1:100: tryptone 10g / L, yeast extract 5g / L, disodium hydrogen phosphate 3.55g / L, phosphoric acid Potassium dihydrogen 3.4g / L, Ammonium chloride 2.68g / L, Sodium sulfate 0.71g / L, Magnesium sulfate heptahydrate 0.493g / L, Ferric chloride hexahydrate 0.027g / L, Glycerin 5g / L, Glucose 0.3g / L, add kanamycin to 50mg / L. Cultivate at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com