Intelligent controlled-release drug-loaded bone cement and preparation method and application thereof
A bone cement and drug-loading technology, which is applied in the fields of pharmaceutical formulation, drug delivery, and medical science, can solve problems such as sudden release of drugs, "burst release of antibiotics, waste, etc., and achieve simple and easy preparation methods, prevent premature release, Improve the effect of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
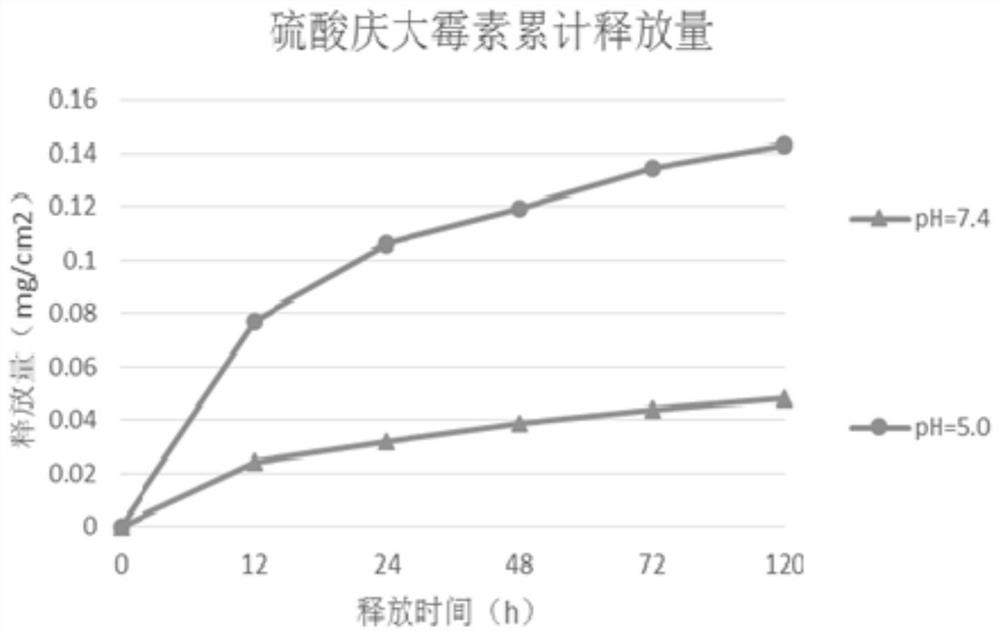
Image
Examples
preparation example Construction
[0091] According to the second aspect of the present invention, there is provided a method for preparing the above intelligent drug-loaded bone cement, comprising the following steps:
[0092] (a) preparing hollow polydopamine nanoparticles / antibiotic drug-loaded microspheres;
[0093] (b) mixing polymethyl methacrylate, initiator, hollow polydopamine nanoparticles / antibiotic drug-loaded microspheres and developer according to the proportioning ratio to obtain a powder;
[0094] (c) Intelligent drug-loaded bone cement composed of powder and liquid.
[0095] Further, step (a) comprises the following steps:
[0096] Dissolving the hollow polydopamine nanoparticles in buffer solution containing antibiotics to avoid light reaction, separating and washing to obtain hollow polydopamine nanoparticles / antibiotic drug-loaded microspheres;
[0097] Preferably, the antibiotic concentration in the buffer solution containing the antibiotic is 0.1-0.3%.
[0098] Taking gentamicin as an e...
Embodiment 1
[0116] An intelligent controlled-release drug-loaded bone cement includes powder and liquid; the liquid-solid ratio of the powder and liquid is 1(ml):2.12(g).
[0117] The powder comprises the following components in mass percentage: 79.5% of polymethyl methacrylate, 0.5% of benzoyl peroxide, 10.0% of hollow polydopamine nanoparticles / microspheres loaded with antibiotics, and 10.0% of barium sulfate.
[0118] The solution includes the following components in mass concentration: 98% of methyl methacrylate monomer, 2% of N,N-dimethyl-p-toluidine, and 70ppm of hydroquinone.
Embodiment 2
[0120] An intelligent controlled-release drug-loaded bone cement includes powder and liquid; the liquid-solid ratio of the powder and liquid is 1(ml):2.15(g).
[0121] The powder comprises the following components in mass percentage: 78.5% of polymethyl methacrylate, 0.6% of benzoyl peroxide, 11% of hollow polydopamine nanoparticles / microspheres loaded with antibiotics, and 9.9% of barium sulfate.
[0122] The solution includes the following components in mass concentration: 97.5% of methyl methacrylate monomer, 2.5% of N,N-dimethyl-p-toluidine, and 60 ppm of hydroquinone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com