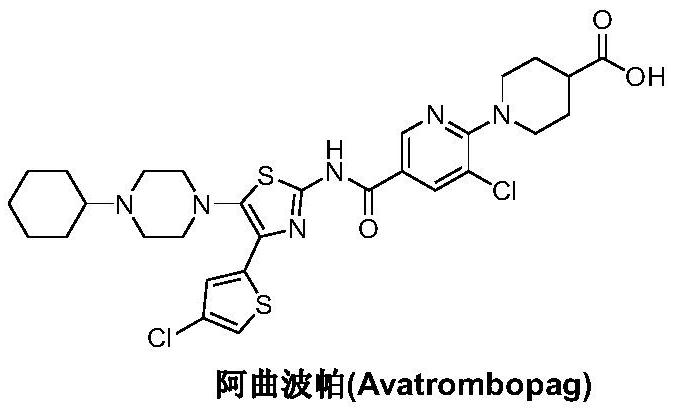
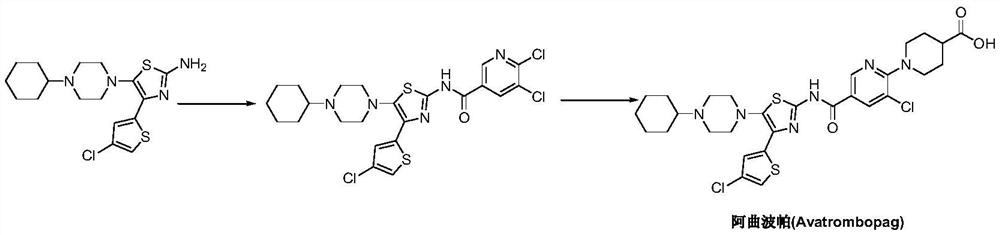
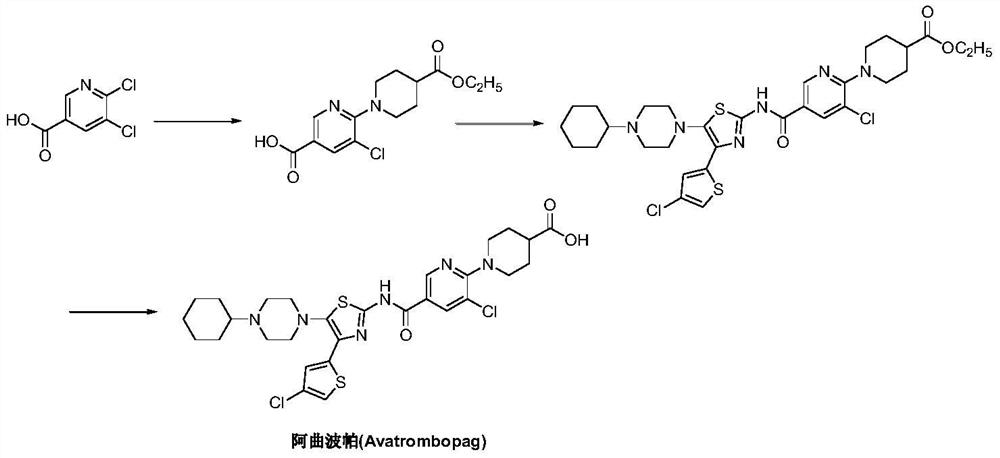
Preparation method of avatrombopag
A technology of atrobolism and cyclization reaction, which is applied in the field of organic synthesis route design and the preparation of raw materials and intermediates, can solve the problems of unavailable raw materials, affecting product quality and yield, and many reaction steps. Large-scale industrial production, the effect of overcoming the poor selectivity of dichloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Under nitrogen protection, add 6-amino-5-chloro-3-pyridinecarboxylic acid (II) (1.72g, 10mmol), condensing agent benzotriazol-1-yloxy tris(dimethylamino ) phosphonium hexafluorophosphate (BOP) (6.63 g, 15 mmol) and acetonitrile 100 mL. Under stirring, the base accelerator 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) (2.28g, 15mmol) was added, the temperature was raised to 60-70°C, and the reaction was carried out for 12 hours. 4-(4-Chloro-2-thienyl)-5-(4-cyclohexyl-1-piperazinyl)-2-thiazolamine (III) (4.21 g, 11 mmol) was added, and the stirring reaction was continued for 12 hours. Cool to room temperature, quench the reaction with saturated brine, and adjust the pH to 4-5 with dilute hydrochloric acid. Concentrate under reduced pressure, and add ethyl acetate to extract the residue 3 times. The organic phases were combined, washed successively with pure water and brine, dried, and the solvent was recovered by distillation under reduced pressure. The obtained oily residu...
Embodiment 2
[0031] Add N-[4-(4-chloro-2-thienyl)-5-(4-cyclohexyl-1-piperazinyl)-2-thiazolyl]-6-amino-5-chloro- 3-Pyridinecarboxamide (IV) (2.69g, 5mmol), sodium lauryl sulfate (67mg, 2.5%w / w), sodium bicarbonate 0.92g, 11mmol) and water 500mL, after stirring at room temperature for 30 minutes, add 4-Bromo-2-(2-bromoethyl)butanoic acid (2.06g, 7.5mmol), heated up to 80-85°C, and reacted for 4-6 hours. It was filtered, extracted three times with dichloromethane, washed with saturated brine and water successively, dried and concentrated. The resulting residue was recrystallized from ethyl acetate to obtain 3.05 g of atrombopag (I), with a yield of 94.0%, EI-MS m / z: 650 [M+H] + ; 1H NMR(DMSO-d6)δ12.68(s,1H),12.29(brs,1H),10.50(d,J=1.6Hz,1H),8.83(d,J=1.6Hz,1H),8.40(d ,J=1.6Hz,1H),7.57(s,1H),7.49(s,1H),7.26(s,1H),3.98(m,2H),3.60(m,2H),3.34(m,7H) ,2.50(m,1H),2.17(m,2H),1.93(m,7H),1.66(m,5H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com