Immunomodulatory protein mutant and nucleotide sequence thereof, recombinant plasmid vector, engineering bacterium, construction method and application
An immunomodulatory protein and nucleotide sequence technology, applied in the field of genetic engineering, can solve the problems of poor stability and inability to further improve the thermal stability of recombinant LZ-8
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Construction of Ganoderma lucidum immunomodulatory protein site-directed mutants
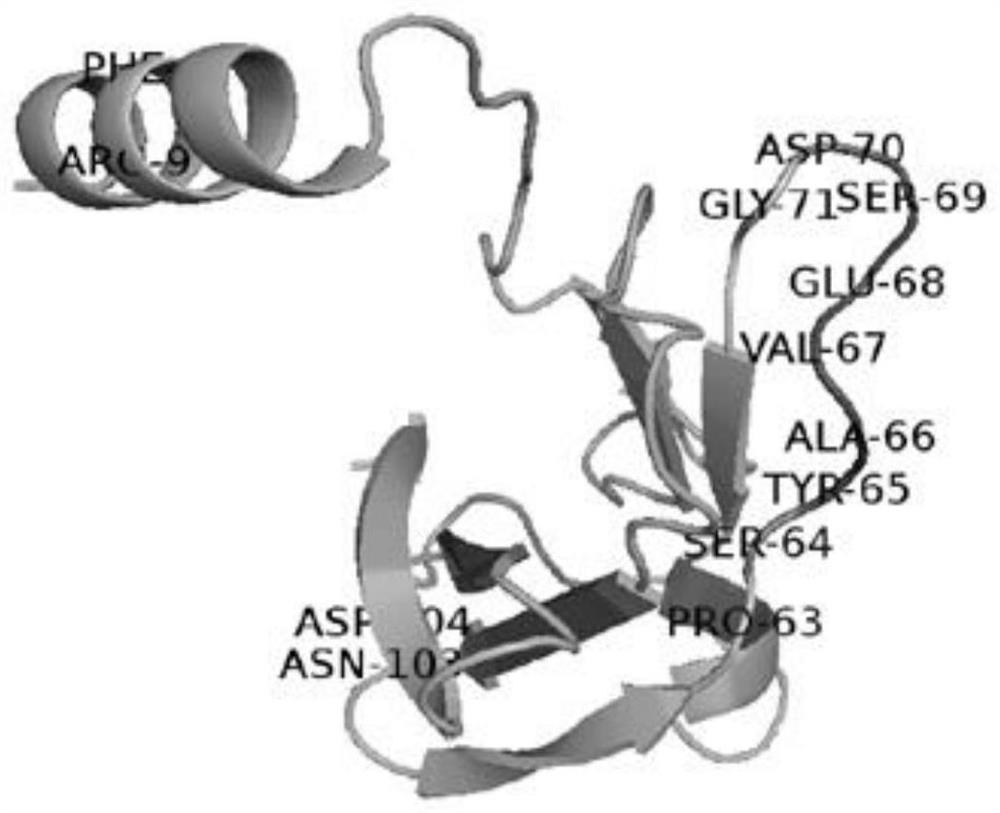
[0052] Such as figure 2 , where the letter indicates the name of the amino acid residue in the thermosensitive region, and the number indicates the order of the amino acid residue in the amino acid sequence. On the N-terminal α-helix of the LZ-8 molecule, the 8th and 9th amino acid residues If the B-factor factor value of the base is higher than that of amino acid residues at other positions, the present invention selects the 8th amino acid residue for a single point mutation, and compares it with the selection of the 9th amino acid residue for single point mutation and simultaneously selects the 8th and 8th amino acid residues. The double-site mutations of the 9 amino acid residues were compared, and the construction of the three mutants were named in turn: F8W, R9K and DM.
[0053] According to the sequence of LZ-8 (the amino acid sequence is shown in SEQ ID NO.1, and the n...
Embodiment 2
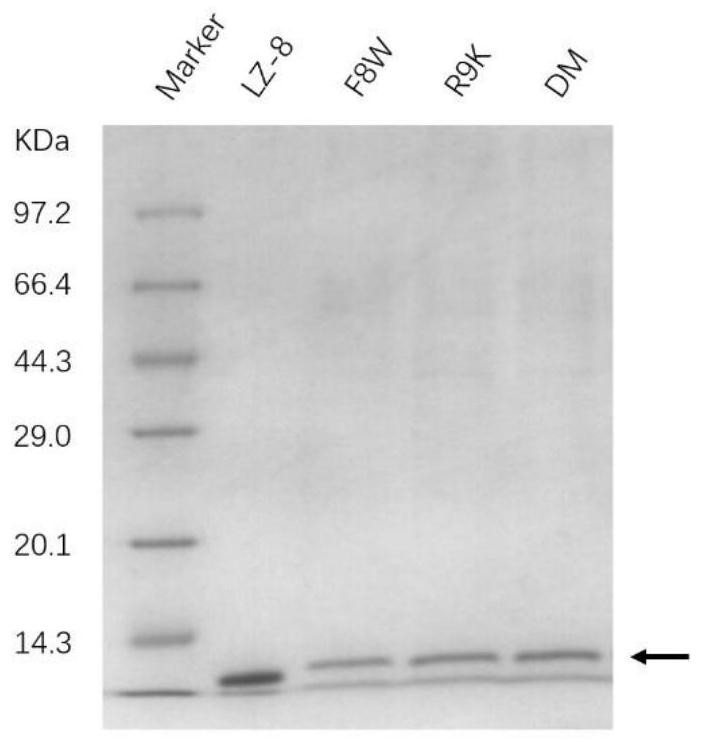
[0057] Example 2: Expression and purification of Ganoderma lucidum immunomodulatory protein mutants
[0058] Pick the yeast X-33 strain expressing the mutant protein in YPD liquid medium (containing 100 μ g / ml Zeocin TM ) at 28-30°C for overnight shaking culture (250-300rpm); the next day, according to the inoculum size of 5%, the seed fermentation liquid was connected to a fermenter containing YPD liquid medium, and after continuous culture and fermentation at 28-30°C for 72 hours, 4°C The supernatant of the fermentation broth was collected by centrifugation.
[0059] AKTA protein purifier was used to purify the recombinant protein, and the temperature of the whole purification process was controlled at 4°C. The specific steps are as follows: (1) SP Sapharose XL cation exchange chromatography to remove most of the miscellaneous proteins and small molecular nucleic acid fragments, pigments, etc., A phase pH3.5 50Mm NaAc-HAc, B phase pH3.5 50Mm NaAc-HAc / 1M NaCl Linear elution...
Embodiment 3
[0060] Example 3: Determination of Immunomodulatory Activity
[0061] The mouse spleen lymphocyte proliferation assay was used to detect the immunomodulatory activity of the mutant, and the specific operation method was as follows: the mouse spleen was isolated by aseptic surgery, a single splenocyte was isolated by ophthalmic shearing, a single splenocyte was filtered through a 200-mesh filter, and red blood cells were lysed. After the action of the solution, collect the remaining cells, count the cell density, dilute the cells to an appropriate density with 1640 culture medium containing 2% fetal bovine serum, add 1×10 5cells, then add the protein to be detected at a predetermined final concentration, and place at 37°C in 5% CO 2 After culturing in an incubator for 24 hours, the cell proliferation was detected by the CCK-8 method ( Figure 4 ). The results showed that LZ-8 and its mutant proteins had consistent splenocyte proliferation-promoting effect curves, and their ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com