Lithium ion battery electrolyte additive cyclic ethylene carbonate sulfate and preparation method thereof
A technology of ethylene carbonate sulfate and electrolyte additives, which is applied in the field of lithium-ion batteries, can solve the problems of incomplete reaction between polyols and carbonates and the impact on battery performance, and achieve the effects of high product purity, high production efficiency, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
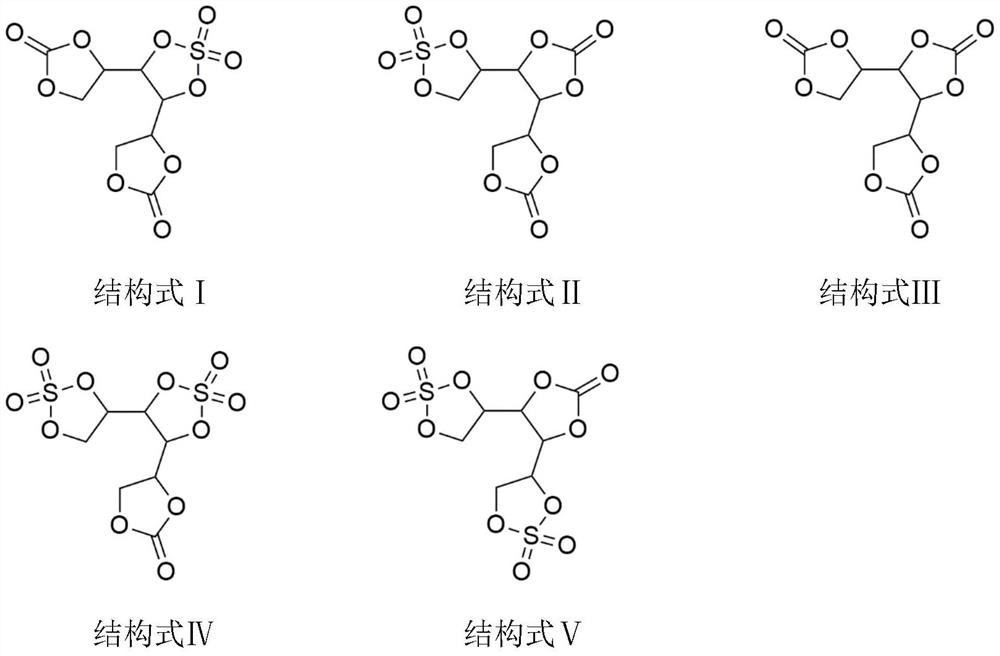
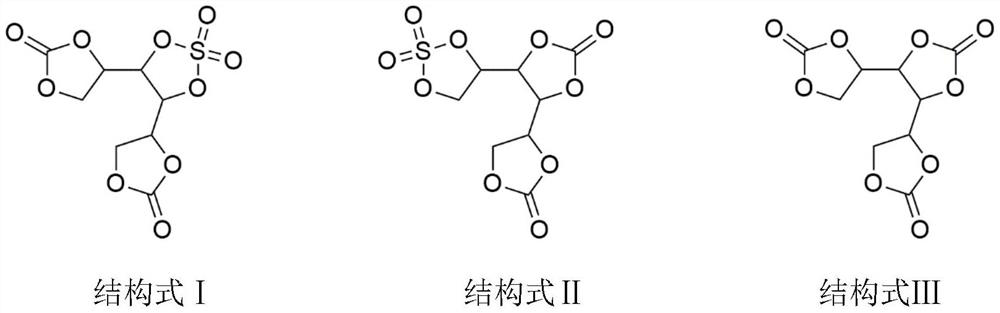
[0021] In the 1000mL three-necked flask, drop into galactitol 91.1g, then drop into it diethyl carbonate (reactant) 118.1g, acetonitrile 300g, and DBU 0.09g, put into stirring bar, three-necked flask is placed in oil bath, heat up To 50°C, fully stirred and reacted under negative pressure of 2000Pa, the reaction time was 3h, the mass of ethanol collected in the water separator was 180.1g, and the reaction was completed. Add 67.5g of sulfuryl chloride, react for 2 hours, use an oil pump to evacuate, and keep the vacuum at 2000Pa for 2 hours to remove excess sulfuryl chloride and hydrogen chloride gas. Then stop heating and cool down to room temperature. Add 500 g of diethyl ether for recrystallization, filter under reduced pressure to obtain a white solid, and vacuum-dry at 40°C for 12 hours to obtain 136.8 g of the product of structural formula I or II, with a yield of 92.1% and a product purity of 99.65%.
Embodiment 2
[0023] Put 91.1g of mannitol into a 1000mL three-necked flask, then put 90.8g of dimethyl carbonate, 300g of DMF, and 0.9g of triethylamine into it, put a stirring bar, put the three-necked flask in an oil bath, and raise the temperature to 60°C. Fully stir the reaction under the condition of negative pressure 1000Pa, the reaction time is 5h, the mass of methanol collected in the water separator is 128.7g, and the reaction is completed. Add 67.5g of sulfuryl chloride, react for 4 hours, use an oil pump to vacuum, and keep the vacuum at 1000Pa for 2 hours to remove excess sulfuryl chloride and hydrogen chloride gas. Then stop heating and cool down to room temperature. Add 500 g of n-octane for recrystallization, filter under reduced pressure to obtain a white solid, and vacuum-dry at 50°C for 12 hours to obtain 139.9 g of the product of structural formula I or II, with a yield of 94.2% and a product purity of 99.88%.
Embodiment 3
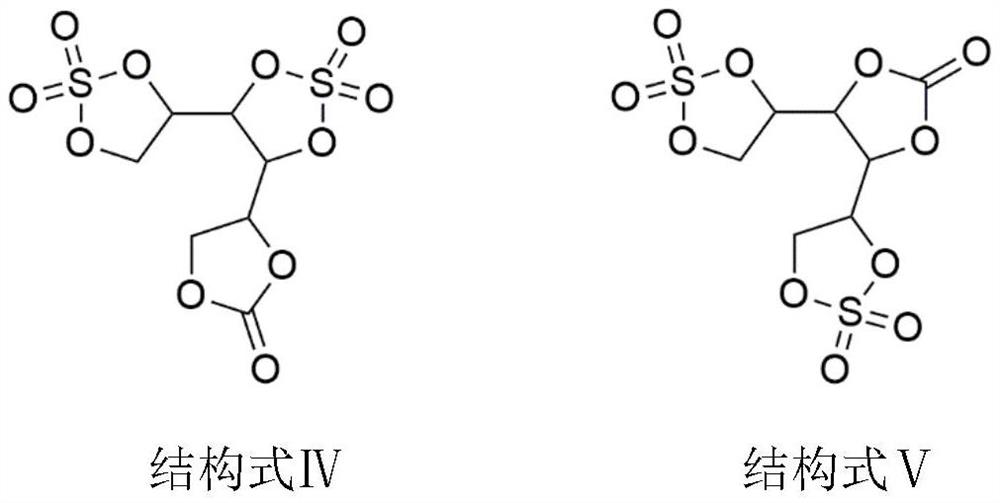
[0025] Put 91.1g of mannitol into a 1000mL three-necked flask, then drop 44.1g of ethylene carbonate, 500g of dimethyl sulfoxide, and 1.8g of DMAP into it, put a stirring bar, place the three-necked flask in an oil bath, and heat up to 70 °C, under the condition of negative pressure of 500Pa, fully stir the reaction, the reaction time is 6h, the mass of ethylene glycol collected in the water separator is 66.8g, and the reaction is completed. Add 102.6g of sulfuryl fluoride, react for 6 hours, use an oil pump to vacuum, and keep the vacuum at 500Pa for 2 hours to remove excess sulfuryl chloride and hydrogen chloride gas. Then stop heating and cool down to room temperature. Add 500 g of methyl ethyl ether for recrystallization, filter under reduced pressure to obtain a white solid, and vacuum-dry at 60°C for 12 hours to obtain 164.8 g of the product of structural formula IV or V, with a yield of 99.0% and a product purity of 99.92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com