Use of Dasainib and Quercetin pharmaceutical composition in preparing medicine for preventing and/or treating side effects of glucocorticoid
A technology of glucocorticoid and dasatinib, applied in the field of medicine, can solve the problem of eliminating the side effects of glucocorticoid without dasatinib and quercetin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Construction of animal model
[0051] 1. Establishment of mouse PDE model
[0052] Pregnant mice were treated with dexamethasone during the 12th to 14th day of pregnancy to establish a prenatal application of dexamethasone (PDE) model. As briefly outlined below, 8-12 week old C57BL / 6 female mice were mated with male mice overnight. The day when the vaginal suppository appeared was set as GD (gestational day) 0, and the pregnant mice were randomly divided into PDE group or control group. Dexamethasone sodium phosphate (Cat.2392-39-4, "Tianxin") was given subcutaneously (1.2 mg / kg / d) during GD 12-14 to establish a PDE mouse model, and this group of mice was called the model group . To establish a control for the PDE model, during GD 12-14, a control group of pregnant mice was treated with daily subcutaneous injections of the same amount of solvent (normal saline), and this group of mice was called the control group. In order to avoid data bias caused by ...
Embodiment 2
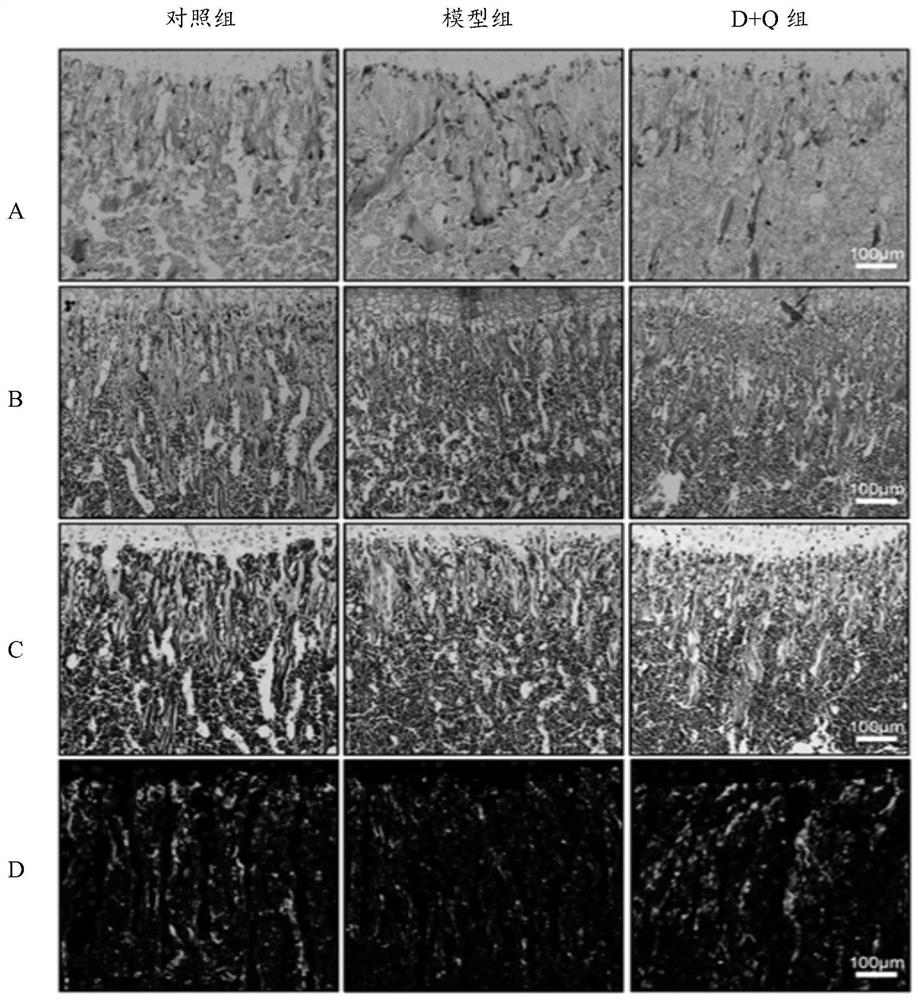
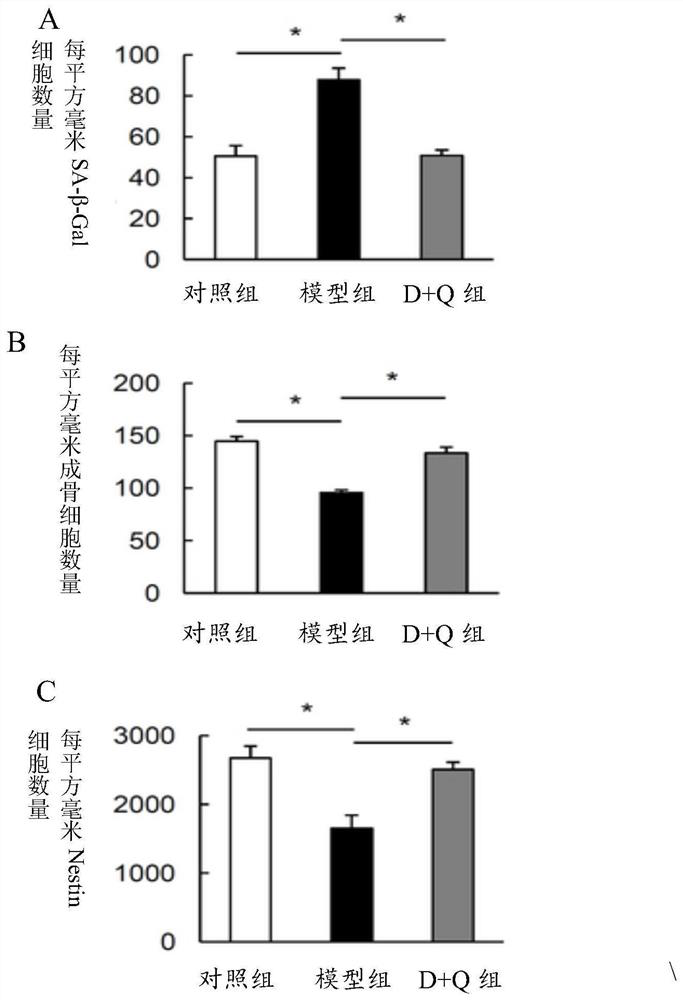
[0055] Embodiment 2: Study on bone morphology of progeny mice
[0056] 1. Specimen fixation and decalcification
[0057] The offspring mice were killed by cervical dislocation after anesthesia, and the femur and tibia were taken out, respectively fixed in 4% paraformaldehyde, decalcified with 0.5M ethylenediaminetetraacetic acid (EDTA, pH 7.4), and passed through a gradient concentration of ethanol / 30% Paraffin-embedded / frozen-embedded after sucrose dehydration, so as to study the growth morphology of the long bones of offspring mice.
[0058] 2. Senescence-associated β-galactosidase (SA-β-Gal) staining
[0059] SA-β-Gal staining steps:
[0060] (1) Frozen sections were thawed in a 37°C oven for 30 minutes.
[0061] (2) Prepare β-Galactosidase staining solution: 930 μL staining solution + 50 μL X-gal solution (20 mg / mL, store at -20°C in the dark) + 10 μL A solution + 10 μL B solution.
[0062] (3) The slices were washed once with PBS, 5 minutes each time.
[0063] (4) Ad...
Embodiment 3
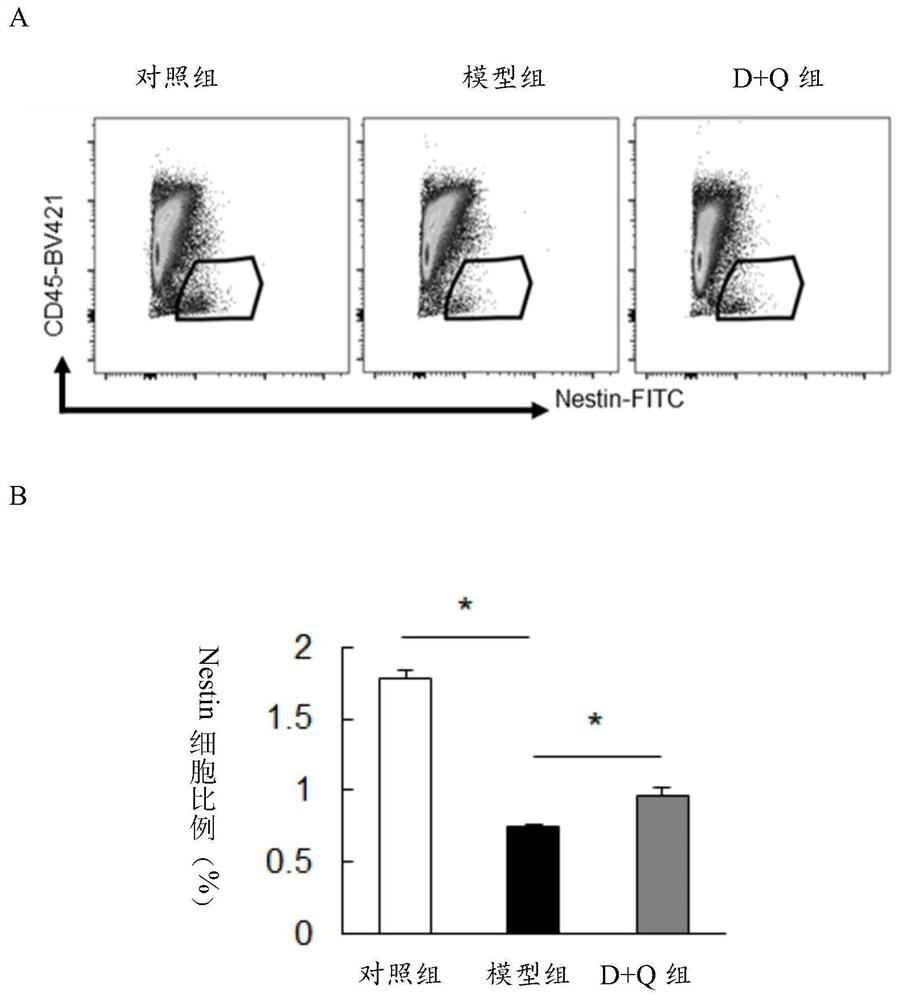
[0116] Flow cytometric analysis and statistical analysis of embodiment 3PDE progeny mice
[0117] Flow Cytometry
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com