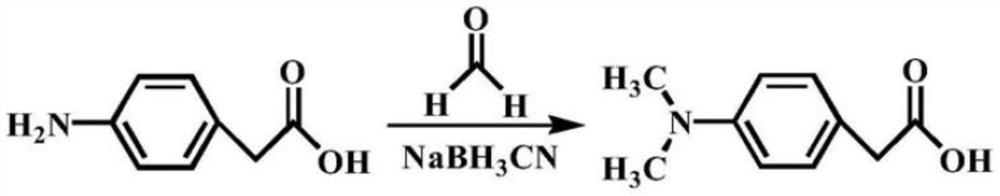
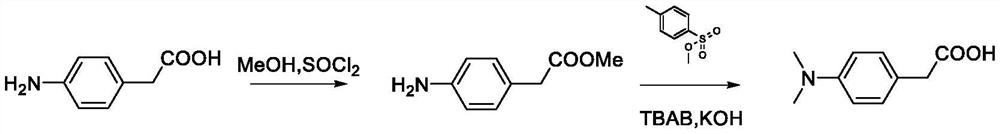
Synthetic method of poly-substituted dimethyl amino phenyl acetic acid compound
A technology of dimethylaminophenyl and synthetic methods, which is applied in the field of organic compound synthesis, can solve the problems of complex operation, poor safety, and harm to skin and mucous membranes, and achieve the effects of good safety, mild reaction, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of 2-(4-(dimethylamino)phenyl)acetic acid
[0048]
[0049] The synthesis steps are: (1) add 2-(4-aminophenyl)acetic acid (4.53g, 30mmol), 25mL of anhydrous methanol, SOCl to a 100mL flask 2 (2.20mL, 30mmol), stirred at 60°C for 12h, cooled to room temperature, and removed the solvent by rotary evaporation to obtain intermediate 1 (methyl 2-(4-aminophenyl)acetate), which was carried out to the next step.
[0050](2) Under Ar protection conditions, add 2-(4-aminobenzene) methyl acetate (1.65g, 10mmol) and methyl p-toluenesulfonate (7.45g, 40mmol) in a 50mL flask, and add 2N KOH solution (30mL), heated to 80°C and stirred, quickly added tetrabutylammonium bromide (0.13g, 0.4mmol), reacted for 6h, cooled the reaction solution to room temperature, extracted with ether, combined the organic phases, and dried over anhydrous magnesium sulfate treatment, spin off most of the solvent, and purify by silica gel column chromatography, using ethyl acetate / petroleum eth...
Embodiment 2
[0054] Synthesis of 2-(4-(dimethylamino)-3-methylphenyl)acetic acid
[0055]
[0056] In this example, the specific preparation method is the same as in Example 1, except for the following conditions: in step (1), replace 2-(4-amino) with 2-(4-amino-3-methylphenyl)acetic acid (30mmol) phenyl) acetic acid, intermediate 2 is obtained; in step (2), intermediate 2 (10mmol) is taken to replace intermediate 1 (2-(4-aminobenzene) methyl acetate), and product 2-(4-( Dimethylamino)-3-methylphenyl)acetic acid, yield 80%.
[0057] Carry out NMR detection to product, the result is as follows:
[0058] 1 H NMR (400MHz,D 2 O) δ7.15(dt,1H),7.07(q,1H),6.76(d,1H),3.55(t,2H),2.84(s,6H),2.28(s,3H).
[0059] 13 C NMR (125MHz, DMSO) δ177.41(d), 148.99, 130.61(m), 129.03(d), 127.51(ddd), 116.00(d), 43.13, 40.92(dt), 17.97(d).
Embodiment 3
[0061] Synthesis of 2-(4-(dimethylamino)-2-methylphenyl)acetic acid
[0062]
[0063] In this example, the specific preparation method is the same as in Example 1, except for the following conditions: in step (1), replace 2-(4-amino) with 2-(4-amino-2-methylphenyl)acetic acid (30mmol) phenyl) acetic acid, intermediate 3 is obtained; in step (2), intermediate 3 (10mmol) is taken to replace intermediate 1 (2-(4-aminobenzene) methyl acetate), and product 2-(4-( Dimethylamino)-2-methylphenyl)acetic acid, yield 81%.
[0064] Carry out NMR detection to product, the result is as follows:
[0065] 1 H NMR (400MHz,D 2 O)δ7.14(dt,1H),6.71(m,2H),3.59(d,2H),2.95(s,6H),2.21(s,3H).
[0066] 13 C NMR (125MHz, DMSO) δ176.49(d), 151.13, 137.61(d), 130.00(m), 126.06(d), 114.89(q), 110.88(dd), 40.40, 40.10(dd), 19.43( d).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com