Preparation method of acyclovir intermediate N(2),9-diacetyl guanine
A technology for diacetylguanine and intermediates, which is applied in the field of preparation of N,9-diacetylguanine, an intermediate of acyclovir, can solve the problems of high safety risks, low product quality, and large amount of "three wastes". Achieve the effect of low safety risk, low raw material cost and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Dissolve 50g of 2,4-diamino6-hydroxy-5-carboxamidopyrimidine (MW169.14, 0.30mol) in 100g of acetic acid (MW60.05, 1.66mol), slowly raise the temperature to 60-70°C, and control the temperature At 60-70°C, slowly add 86g of 80% acetic anhydride acetic acid solution (MW102.09, 0.67mol) dropwise, and the dropping time is controlled at 6h.
[0055] After dropping, begin vacuum distillation to reclaim the acetic acid solvent. After concentrating to dryness, about 60.9 g of the concentrated residual solid is obtained, which is 2-acetylamino-4-amino-6-hydroxyl-5-formamidopyrimidine (II). The yield is about 96.1%, and the purity is 98.2%. Recovery of acetic acid can be applied repeatedly.
[0056] Add 5g of catalyst A (sodium acetate) and 300g of xylene to compound (II), start stirring and heating, raise the temperature to 125-135°C, reflux for dehydration reaction for 14h, when no obvious water droplets are produced in the water separator, the reaction ends. Begin to cool do...
Embodiment 2
[0060] Dissolve 50g of 2,4-diamino6-hydroxy-5-carboxamidopyrimidine (MW169.14, 0.30mol) in 120g of recovered acetic acid (MW60.05, 1.66mol), slowly raise the temperature to 60-70°C, and control At a temperature of 60-70°C, slowly add 85g of 85% acetic anhydride acetic acid solution (MW102.09, 0.71mol) dropwise, and the dropping time is controlled at 7h.
[0061] After dropping, begin vacuum distillation to reclaim the acetic acid solvent. After concentrating to dryness, about 60.5 g of the concentrated residual solid is obtained, which is 2-acetylamino-4-amino-6-hydroxyl-5-carboxamidopyrimidine (II). The yield is about 95.5%, and the purity is 98.5%. Recovery of acetic acid can be applied repeatedly.
[0062] Add 5g of catalyst A (tetrabutylammonium bromide) and 300g of recovered xylene to compound (II), start stirring and heating, raise the temperature to 115-125°C, reflux for dehydration reaction for 15 hours, when there are no obvious water droplets in the water separator ...
Embodiment 3
[0064] Dissolve 50g (MW193.16, 0.26mol) of 2-acetylamino-6-hydroxypurine (Ⅲ) and 3g of sodium acetate in 500g of 75% acetic anhydride in acetic acid solution (MW102.09, 3.67mol), start stirring and heating, and heat up to 125-135°C and react for 8 hours.
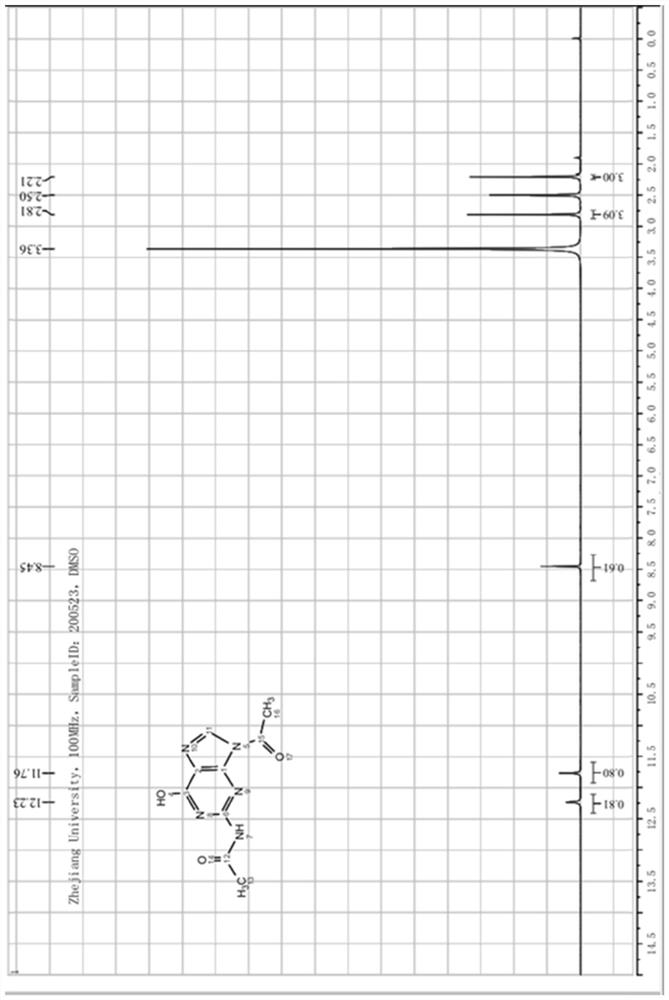
[0065] After the reaction is complete, cool down to 5-15°C, heat and crystallize for 2 hours, filter, and dry to obtain about 59.9 g of off-white crystalline solid powder, which is N(2), 9-diacetylguanine (IV), with a molar yield of 98.0 %, purity 99.1%. After detecting the acetic anhydride content in the filtered mother liquor, it can be applied mechanically directly or after recovering acetic acid by distillation, and then applied mechanically to step (1).
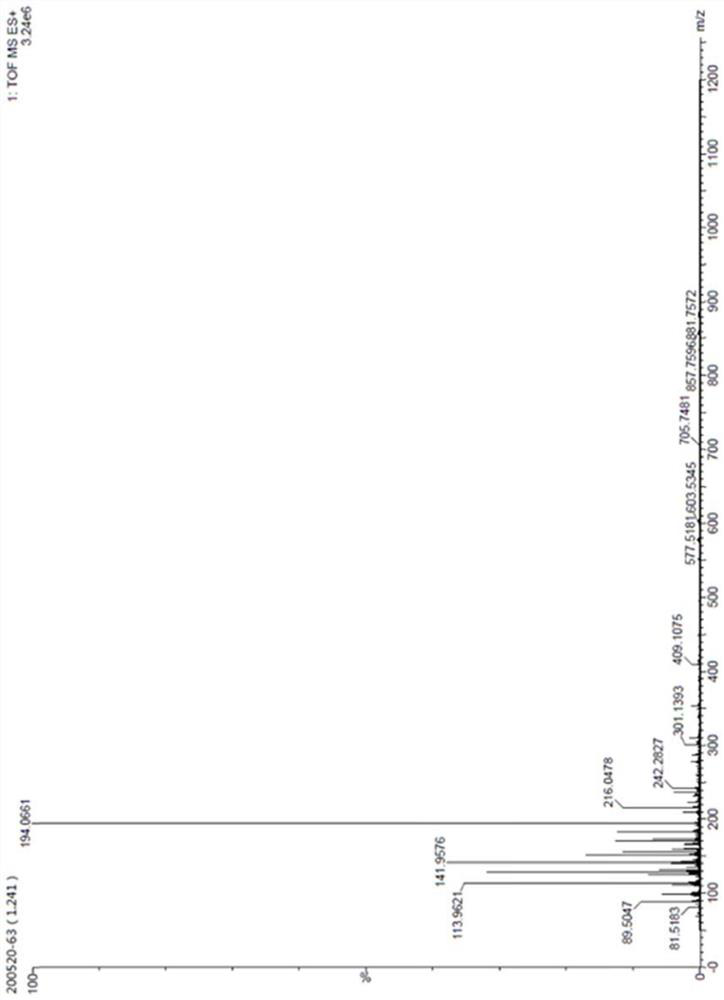
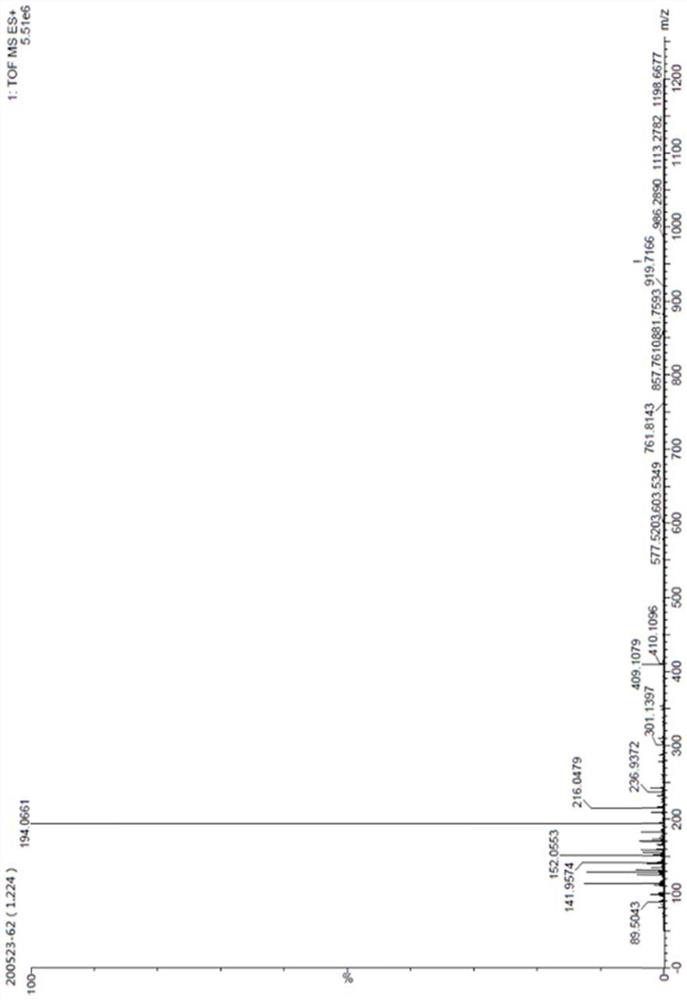
[0066] The N(2),9-diacetylguanine structure analysis data prepared in this example are as follows:
[0067] ESI-MS (M / Z): The ion peak at m / z236 in the ESI-MS positive ion mass spectrum corresponds to the [M+H]+ ion of the sample, and the stronger ion peak at m / z194 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com