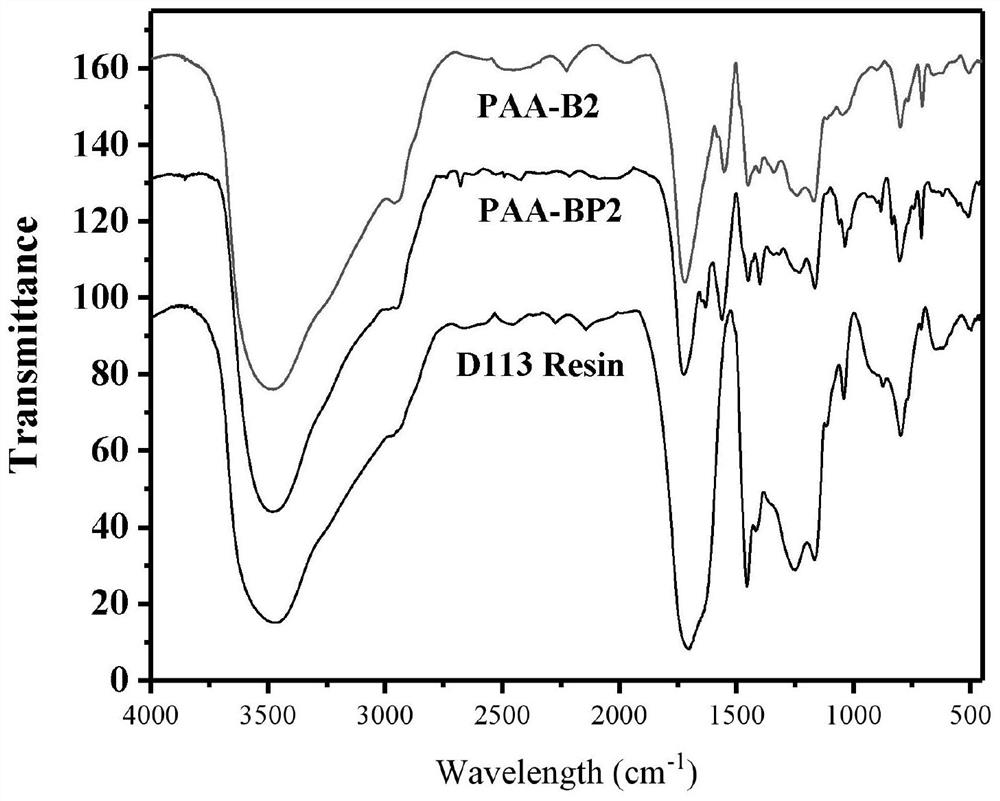
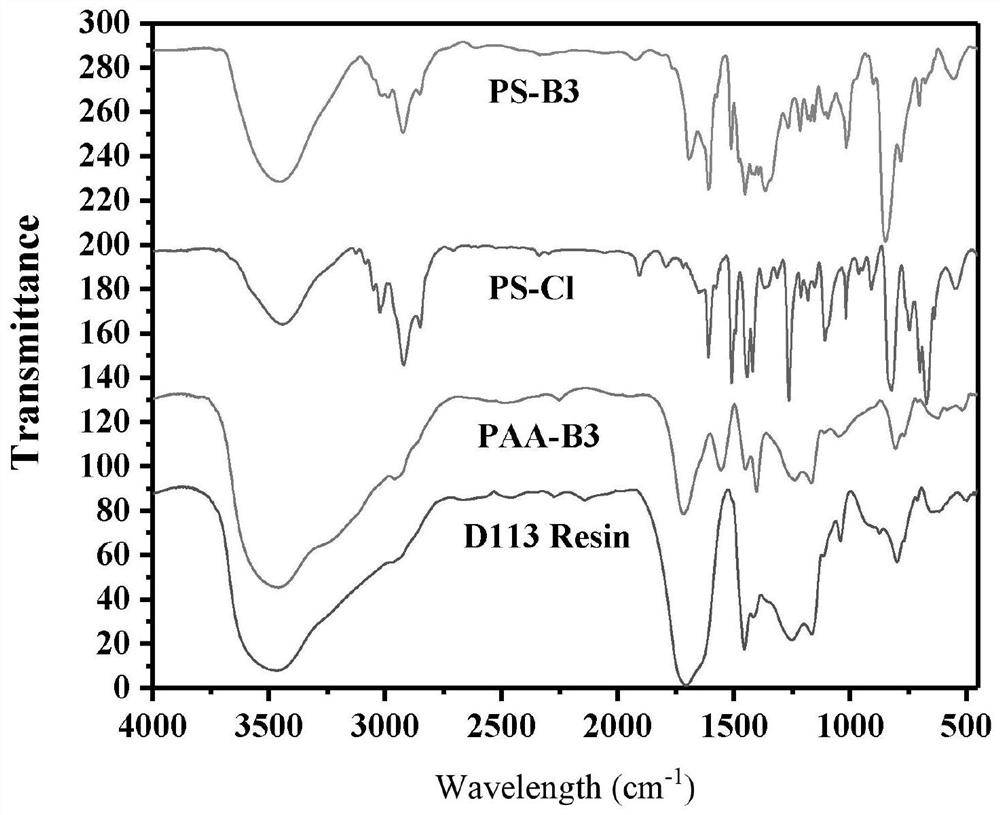
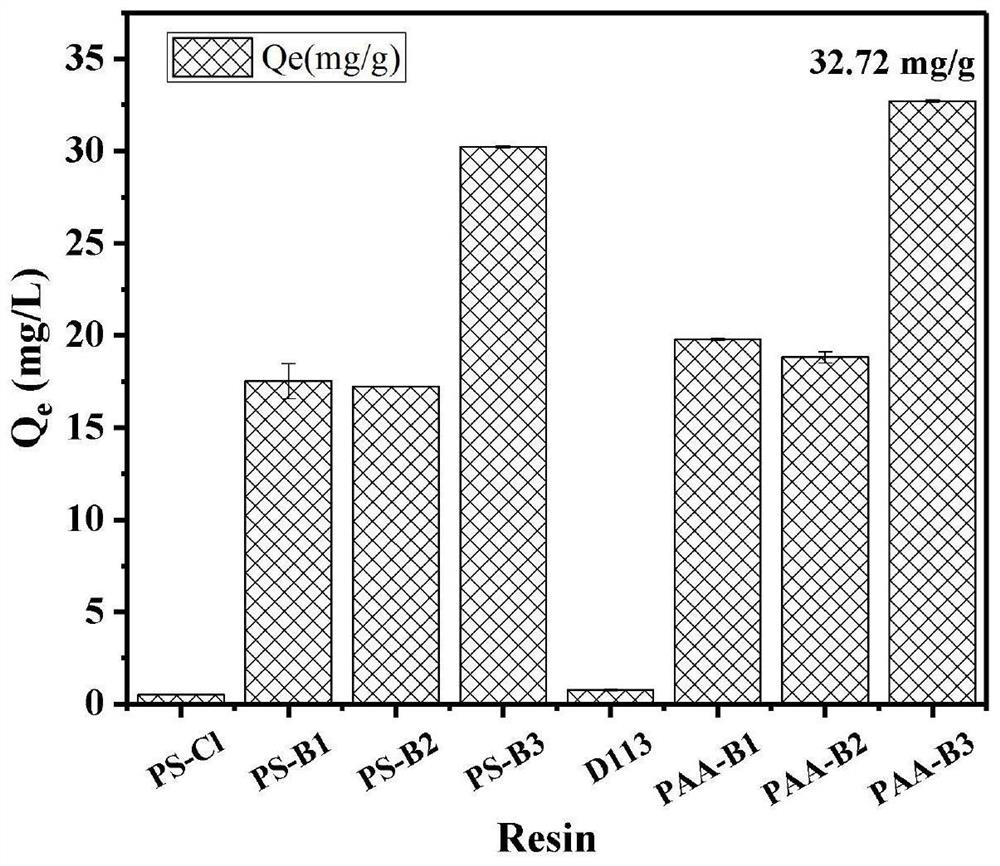
Preparation method of boric acid functional group resin
A technology of functional groups and resins, applied in the field of resins, can solve problems such as the difficulty in efficiently introducing specific boric acid functional groups, low adsorption capacity, and lack of precise control of the material modification process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0067] Specific embodiment one (PS-B1 resin): The preparation method of a boric acid functional resin in this embodiment is carried out in the following steps:
[0068]
[0069] 1. Add 100mL of DMF into a 250mL three-necked flask equipped with a stirrer and a thermometer, add 10g of chloromethyl polystyrene divinylbenzene resin to DMF, and swell for 12 hours at room temperature;
[0070] 2. First, add 3-aminophenylboronic acid (11.40g, 73.6mmol) to the reaction system of step 1, and then add potassium carbonate (10.15g, 73.6mmol), mix well under normal temperature and mechanical stirring, and react at 353K for 24h. After completion, the resin was filtered out, washed with 95% industrial ethanol and water sequentially, washed 4 times each, and dried under vacuum at 313K for 12 hours to obtain PS-B1 resin, which is a boronic acid functional resin.
specific Embodiment approach 2
[0071] Specific embodiment two (PS-B2 resin): The preparation method of a boric acid functional resin in this embodiment is carried out in the following steps:
[0072]
[0073] 1. Add 100mL of DMF into a 250mL three-necked flask equipped with a stirrer and a thermometer, add 10g of chloromethyl polystyrene divinylbenzene resin to DMF, and swell at room temperature for 12 hours;
[0074] 2. First add 3-aminophenylboronic acid pinacol ester (16.12g, 73.6mmol) to the reaction system of step 1, and then add potassium carbonate (10.15g, 73.6mmol), mix well under normal temperature and mechanical stirring, under 353K After the reaction is completed for 24 hours, the resin is filtered out, washed sequentially with 95% industrial ethanol and water, washed 4 times each, and dried under vacuum at 313K for 12 hours to obtain a borate pinacol ester modified resin;
[0075] 3. Put the boric acid pinacol ester modified resin (2g) into a 100mL three-necked flask, then add 30mL methanol and ammoniu...
specific Embodiment approach 3
[0076] Specific embodiment three (PAA-B1 resin): The preparation method of a boric acid functional resin in this embodiment is carried out in the following steps:
[0077]
[0078] 1. Add 100mL of DMF into a 250mL three-necked flask equipped with a stirrer and a thermometer, add 10g of D113 weak acid ion exchange resin to DMF, and swell for 12h at room temperature;
[0079] 2. Firstly add 3-aminophenylboronic acid (14.87g, 96.0mmol) to the reaction system of step 1, and then add HATU (43.77g, 115.2mmol), DIPEA (14.89g, 115.2mmol), and mix at room temperature under mechanical stirring. After the reaction was completed, the resin was filtered out, washed with 95% industrial ethanol and water, 4 times each, and dried under vacuum at 313K for 12 hours to obtain PAA-B1 resin, which is a boronic acid functional resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com