Separation and preparation methods of human amniotic epithelial stem cells
A separation method and stem cell technology, applied in the field of preparation, serum-free culture method and its products, and separation of human amniotic epithelial stem cells, can solve the problems of animal-derived pathogen contamination, foreign protein rejection, amniotic epithelial cell destruction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Enzyme Digestion and Separation Conditions for Human Amniotic Membrane Epithelial Stem Cells
[0064] With the informed consent of the puerpera, the placental amniotic membranes of healthy fetuses who underwent cesarean section before 39 weeks of gestation were collected. After testing, the mother is in good health and has no acute or chronic infectious diseases, family genetic diseases, hepatitis B virus, hepatitis C virus, HIV, human T cell-tropic virus, herpes virus, cytomegalovirus, Treponema pallidum and mycoplasma and other pathogens , The collection of amniotic membrane was carried out under sterile conditions.
[0065] After the amniotic membrane is collected, it is placed in the amniotic membrane preservation solution, transported at 4°C, and arrives at the GMP cell preparation workshop within 12 hours for digestion and separation of human amniotic membrane epithelial stem cells.
[0066] The amniotic membrane was taken out from the preservation solu...
Embodiment 2
[0072] Embodiment 2 Freshly isolated human amniotic membrane epithelial stem cells (Pre-P0) phenotype detection
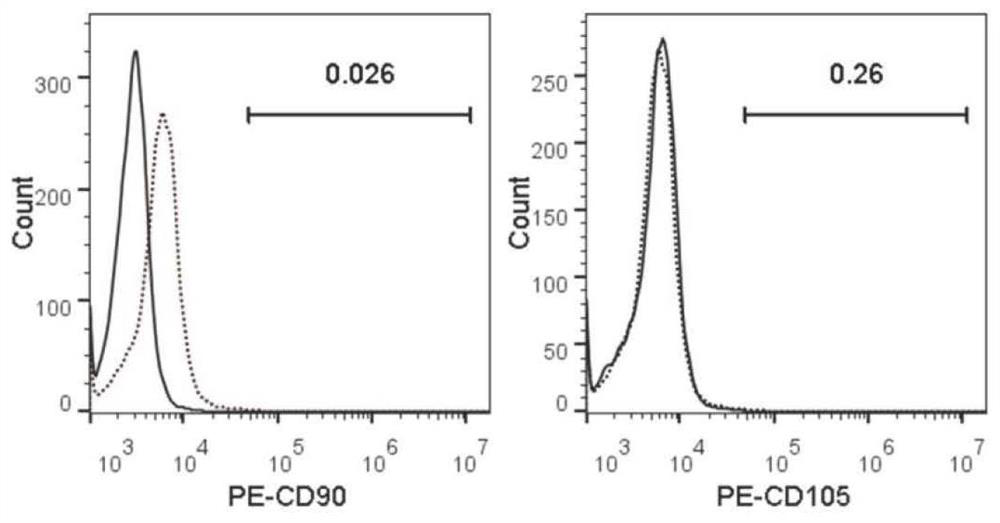
[0073] Pre-P0 cells were separated by trypsinization, and the cells were centrifuged and washed twice with FACS buffer (PBS containing 2% FBS), and the cells were resuspended in FACS buffer, and the cell concentration was adjusted to 1×10 6 / 100μL, split into several tubes, add CD90, CD105 and their respective isotype control antibodies to the cells in each tube, incubate at 4°C in the dark for 30min, centrifuge and wash the cells twice with FACS buffer, resuspend in 500μL FACS buffer Cells, immediately tested on the machine.
[0074] see figure 2 , the results showed that the Pre-P0 cells separated by trypsin digestion did not express two classic mesenchymal stem cell markers, CD90 and CD105, indicating that the isolated human amniotic epithelial stem cells had no mesenchymal stem cell contamination and were of high purity.
Embodiment 3
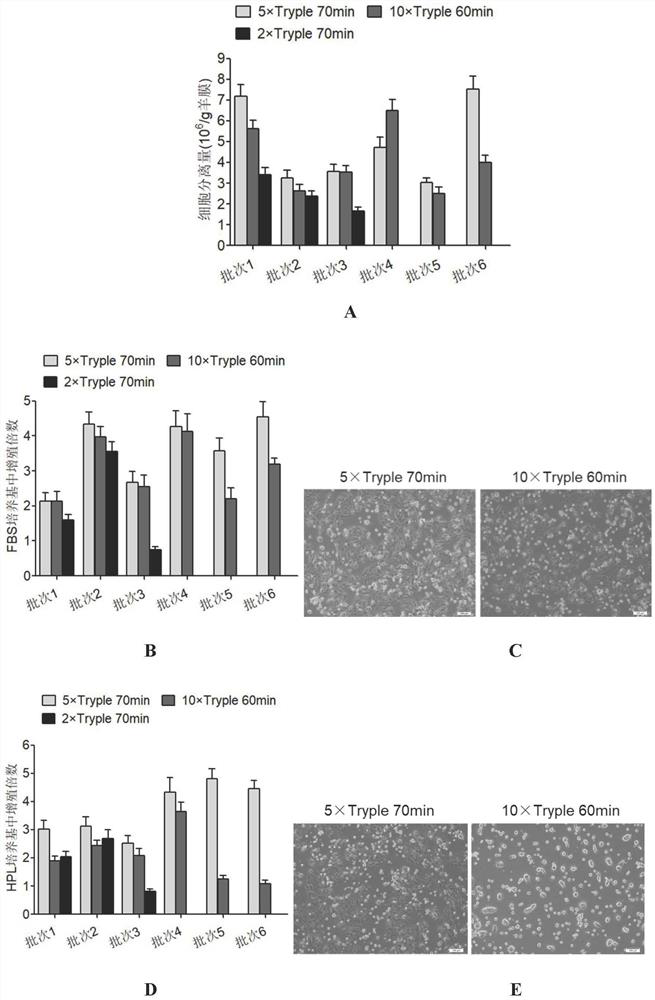
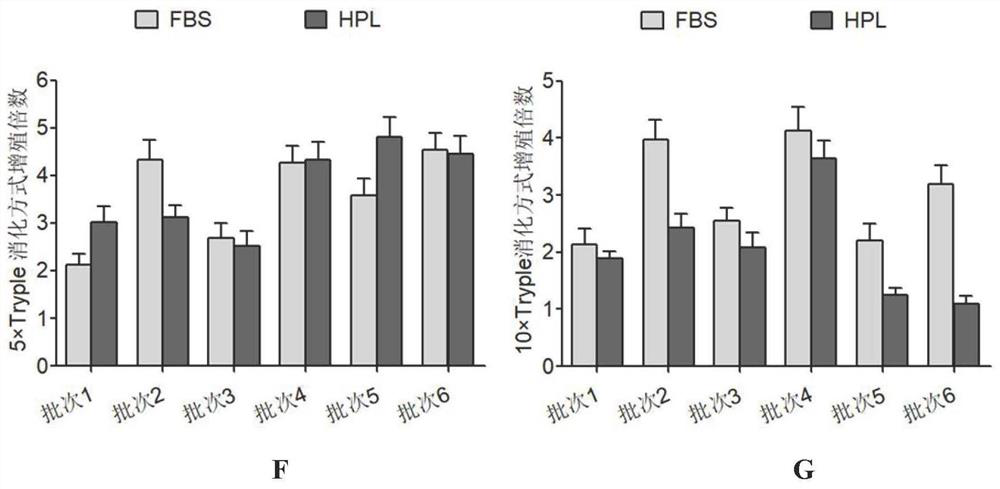
[0075] Example 3 Screening of human amniotic epithelial stem cells in serum-free medium
[0076] The Pre-P0 cells were resuspended in 6 kinds of media, respectively, FBS (DMEM / F-12, 10% concentration of fetal bovine serum, 1mM sodium pyruvate, 1mM non-essential amino acids, 2mM L-glutamine and 10 ng / mL human epidermal growth factor), HPL (DMEM / F-12, 10% platelet lysate, 1mM sodium pyruvate, 1mM non-essential amino acids, 2mM L-glutamine and 10ng / mL human epidermal growth factor), B27 (DMEM / F-12, add 2% volume of Gibco B27supplement, 1mM sodium pyruvate, 1mM non-essential amino acids, 2mM L-glutamine and 10ng / mL human epidermal growth factor), CTS B27 (DMEM / F-12 , add 2% volume of Gibco's CTSB27 supplement, 1mM sodium pyruvate, 1mM non-essential amino acids, 2mML-glutamine and 10ng / mL human epidermal growth factor), N2 (DMEM / F-12, add 1% volume of Gibco company N2supplement, 1mM sodium pyruvate, 1mM non-essential amino acids, 2mM L-glutamine and 10ng / mL human epidermal growth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com