Patents
Literature
56results about How to "Avoid over-standard problems" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
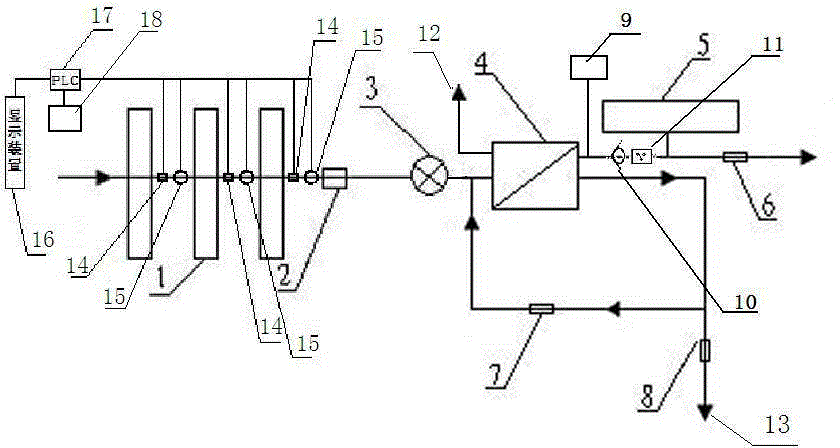
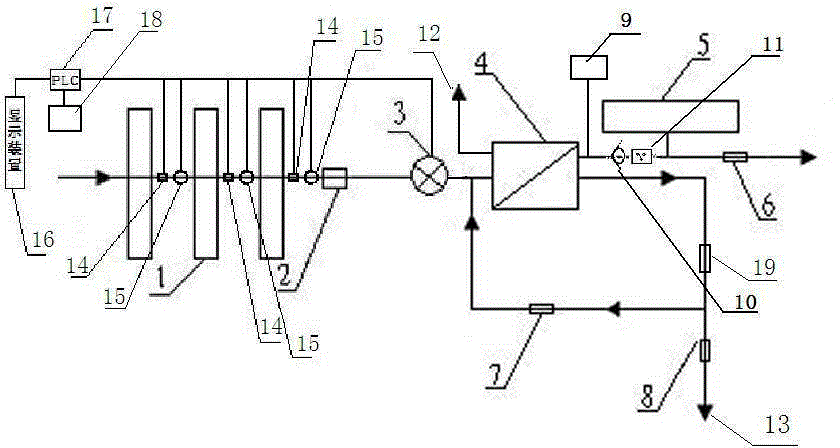
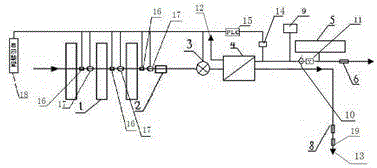
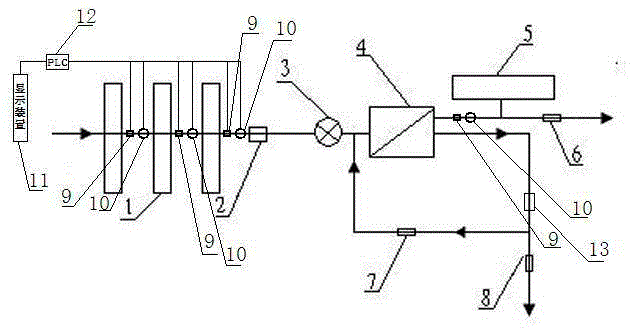
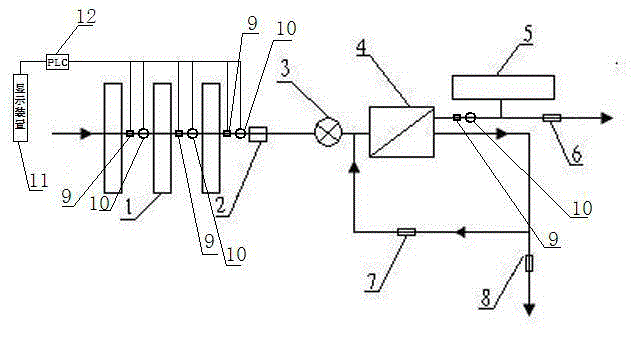
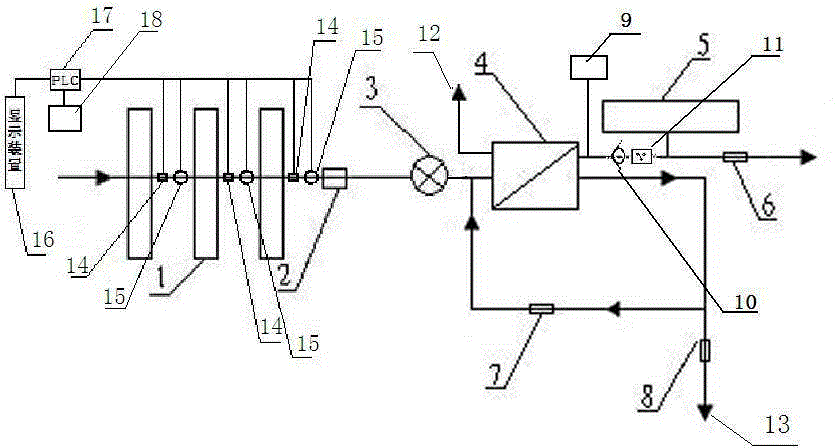
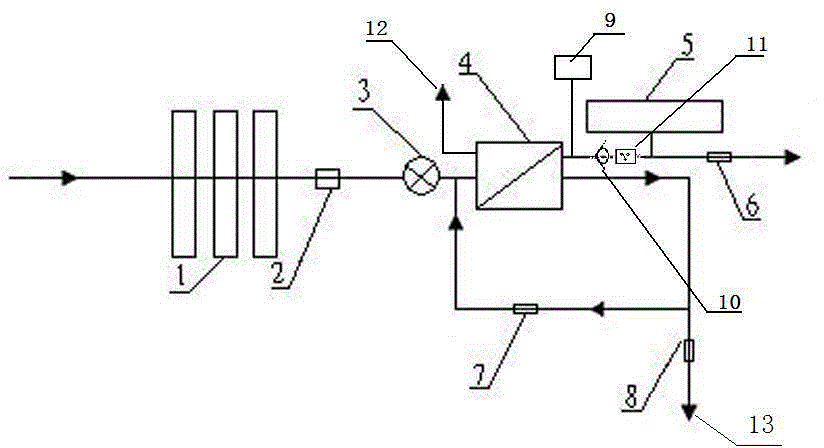
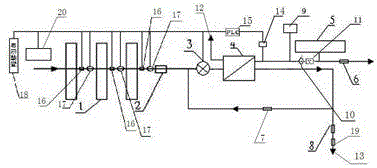
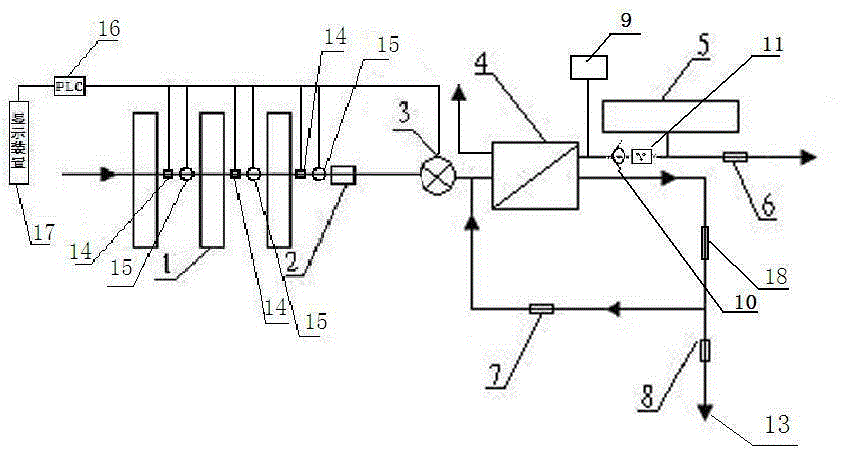
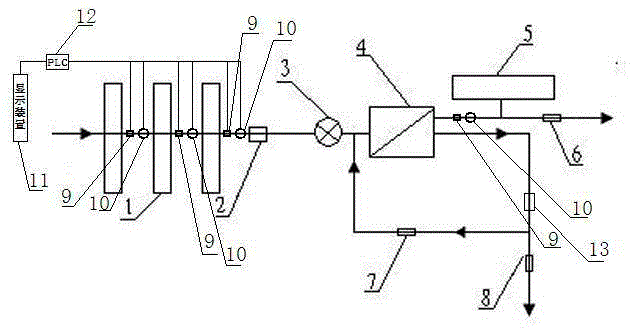
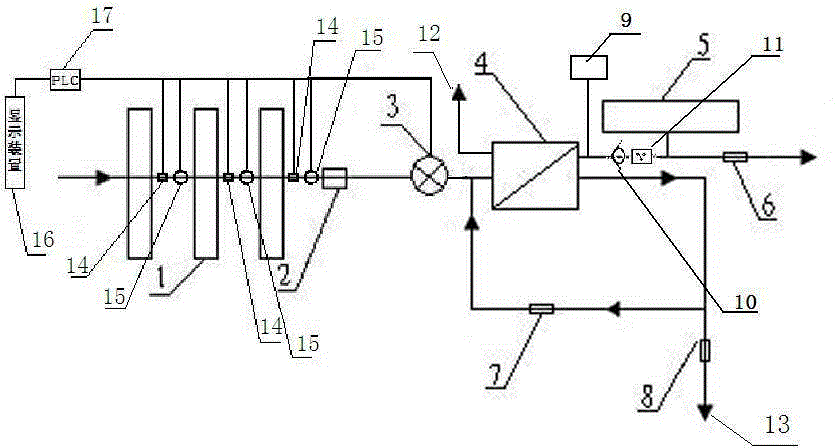
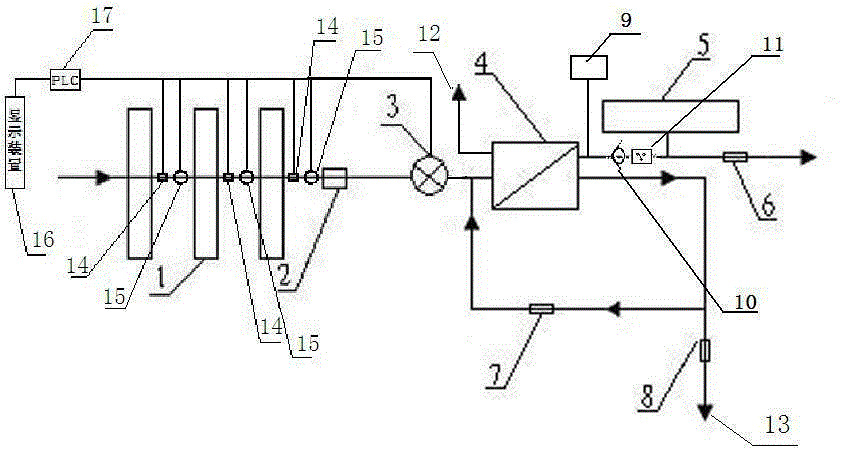
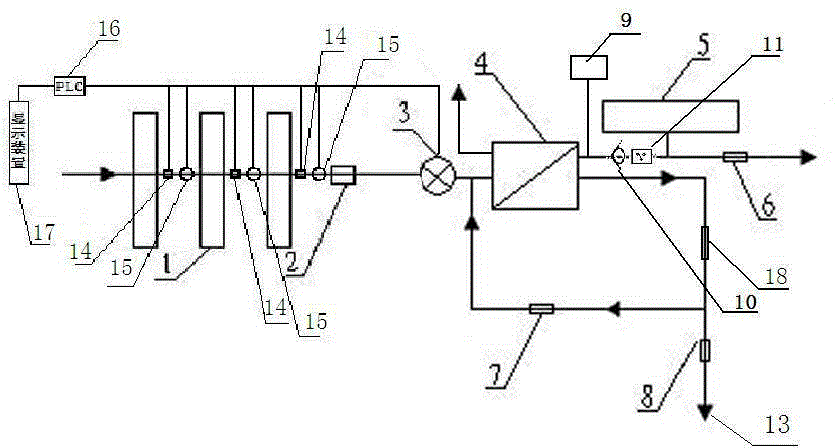
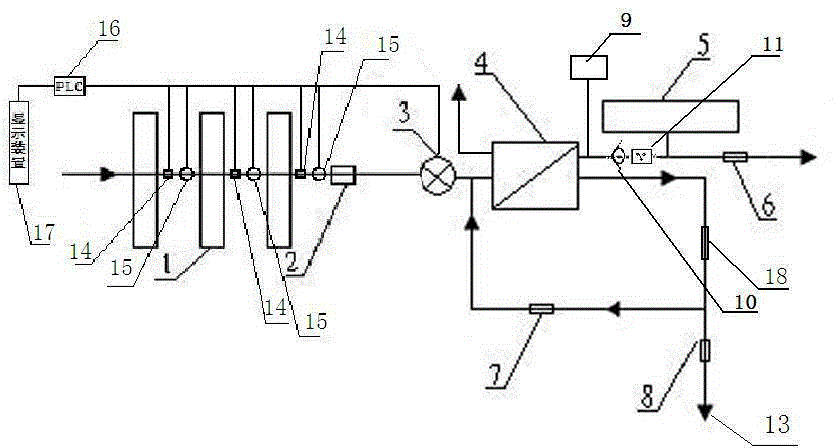
Backwash osmosis water purifier with intelligent monitoring
InactiveCN105540940AExtended service lifeReduce outputReverse osmosisMultistage water/sewage treatmentWater flowWastewater
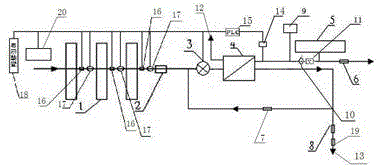
The present invention relates to a backwash osmosis water purifier with intelligent monitoring. According to the present invention, a reverse flushing device and a water flow rate monitoring device are arranged, the water outlet end of a RO reverse osmosis membrane treatment unit is provided with a TDS detection device, a water flow rate sensor and the TDS detection device are connected to a PLC, the PLC is connected to a display device and a network transmission device, the PLC transmits a processed signal to the display device so as to display, and the processed signal is transmitted to terminal equipment through the network transmission device; and the backwash osmosis water purifier has beneficial effects of RO reverse osmosis membrane service life prolonging, energy consumption reducing, and wastewater output reducing.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
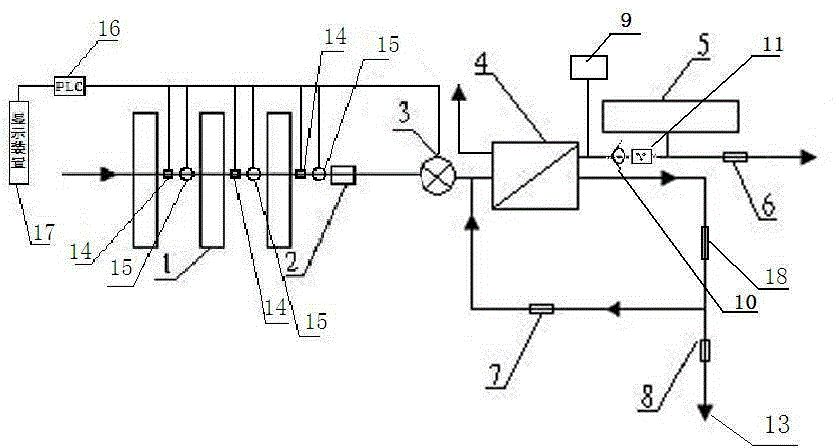
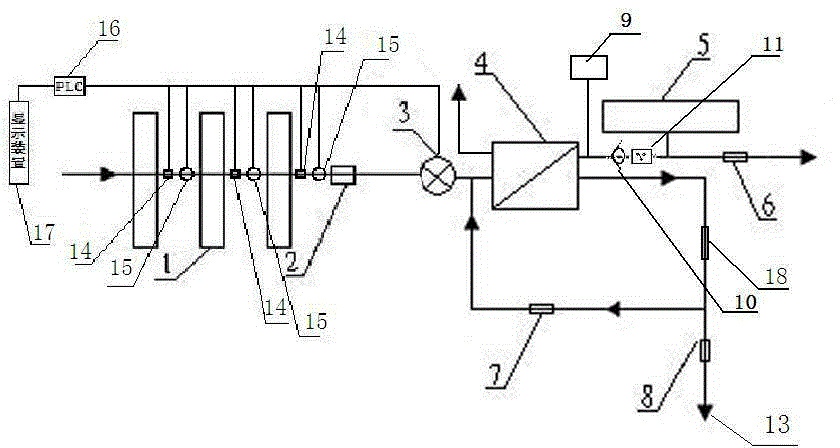
Backwash osmotic water purification machine with monitoring function
InactiveCN105621754AReal-time monitoring of usage statusExtended service lifeReverse osmosisMultistage water/sewage treatmentWater flowDisplay device
The invention relates to a backwash osmotic water purification machine with a monitoring function. According to the invention, a pressure tank and a check valve are connected to the water outlet pipe of an RO reverse osmosis membrane treatment unit; a water flow monitoring device and a TDS detection device are arranged at the water outlet end of each level of filter core of a filtering unit; a wastewater pipe is provided with a wastewater proportioner; the water flow monitoring devices and the TDS detection devices are connected with a PLC, and are connected to a display device through the PLC; the PLC transmits processed signals to the display device; a water pump is connected to the PLC; the PLC controls the on / off of the water pump. With the water purification machine, the service life of the RO reverse osmosis membrane is prolonged, energy consumption is reduced, and wastewater output is reduced.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
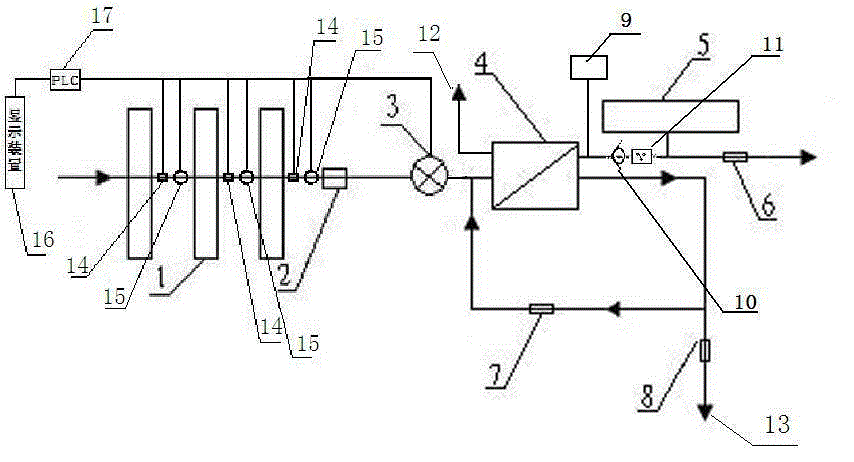
Backwash osmosis water purifier with monitoring
InactiveCN105540941AReduce outputIncrease productivityReverse osmosisMultistage water/sewage treatmentWastewaterReverse osmosis
The present invention relates to a backwash osmosis water purifier with monitoring. According to the present invention, the water inlet end of a RO reverse osmosis membrane treatment unit is provided with a backwash wastewater pipe, the water outlet pipe position of the RO reverse osmosis membrane treatment unit is connected to a pressure tank, and the connection pipeline of the water outlet pipe of the RO reverse osmosis membrane treatment unit and a water storage barrel is connected to a one-way valve; and the beneficial effects of RO reverse osmosis membrane service life prolonging, energy consumption reducing and wastewater output reducing are achieved.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
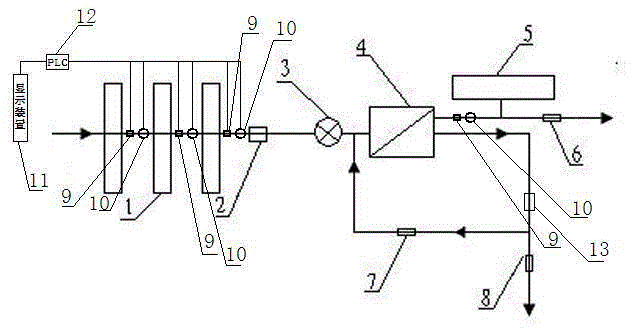
Osmosis water purifier with monitoring
InactiveCN105585173AReal-time monitoring of usage statusExtended service lifeReverse osmosisMultistage water/sewage treatmentWater storageReverse osmosis
The invention relates to an osmosis water purifier with monitoring. The osmosis water purifier includes a water pump, and a filtering unit and a reverse osmosis (RO) membrane treatment unit which are successively connected to a water inlet pipe. A wastewater outlet end of the RO membrane treatment unit is connected to a wastewater pipe which is used for discharging wastewater. A clean water outlet end is connected to a water storage barrel. The water storage barrel is connected to the clean water outlet. An electromagnetic valve is arranged on a water inlet pipe of the RO membrane treatment unit. The filtering unit includes, successively, a PP filter core, a granular activated carbon filter core and a compressed carbon filter core, wherein a water flow quantity monitoring apparatus is disposed at the water outlet end of each filter core in the filtering unit. A TDS detection apparatus is arranged on the water outlet end of the RO membrane treatment unit. The water flow quantity monitoring apparatuses and the TDS detection apparatus are both connected to a display apparatus. The wastewater pipe is also provided with a wastewater proportioner. The osmosis water purifier allows the filtering unit to be monitored timely, increases service life of a RO membrane, reduces energy consumption and reduces wastewater production amount.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
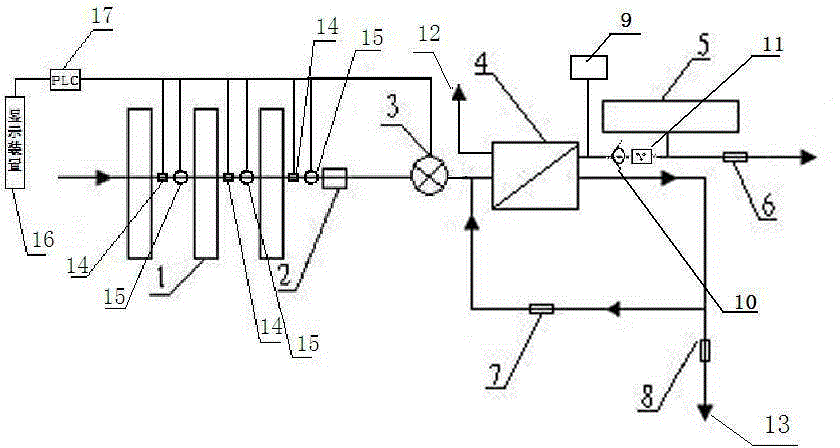
Backwash osmosis water purifier with intelligent monitoring
InactiveCN105540909AExtended service lifeReduce outputSemi-permeable membranesWater/sewage treatment bu osmosis/dialysisIntelligent lightingWastewater
The present invention relates to a backwash osmosis water purifier with intelligent monitoring. According to the present invention, a backwash device and a water flow rate monitoring device are arranged, the water outlet end of a RO reverse osmosis membrane treatment unit is provided with a TDS detection device, a wastewater pipe is further provided with a wastewater proportioner, a water flow rate sensor and the TDS detection device are connected to a PLC, the PLC is connected to a display device and a network transmission device, the PLC transmits a processed signal to the display device so as to be displayed, the processed signal is transmitted to terminal equipment through the network transmission device, and the opening or closing of a water pump is controlled by the PLC; and the beneficial effects of RO reverse osmosis membrane service life improving, energy consumption reducing and wastewater output reducing are achieved.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Timed backwash sterilization osmotic water purification machine with monitoring function
InactiveCN105621684AReal-time monitoring of usage statusExtended service lifeBiocideReverse osmosisWater storage tankReverse osmosis
The invention relates to a timed backwash sterilization osmotic water purification machine with a monitoring function. A backwashing wastewater pipe is arranged at a water inlet end of an RO reverse osmosis membrane treatment unit; a pressure tank is connected at the water outlet pipe of the RO reverse osmosis membrane treatment unit; a check valve is arranged on a pipeline connecting the water outlet pipe of the RO reverse osmosis membrane treatment unit and a water storage tank; a timing device 14 is arranged at the water outlet pipe of the RO reverse osmosis membrane treatment unit; the timing device 14 is connected with a PLC 15; the PLC 15 controls the on / off of a water pump according to signals from the timing device 14; and sterilization materials are adopted to manufacture the water storage tank, water pipes and the like. With the water purification machine, the service life of the RO reverse osmosis membrane is prolonged, energy consumption is reduced, and wastewater output is reduced.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Sterilization monitoring water purification machine
InactiveCN105776627AExtended service lifeAvoid breedingBiocideReverse osmosisSolenoid valveWater storage tank
The present invention relates to a sterilization monitoring water purification machine, a wastewater outlet end of a RO reverse osmosis membrane treatment unit is connected with a wastewater pipe, wastewater is discharged by the wastewater pipe, a purified water outlet end is connected with a water storage tank, the water storage tank is connected with the purified water outlet end, a water inlet pipe of the RO reverse osmosis membrane treatment unit is provided with a solenoid valve, a water outlet end of each stage filter element of a filter unit is respectively provided with a water flow monitoring device, a water outlet end of the RO reverse osmosis membrane treatment unit is provided with a TDS detection device, the water flow monitoring device and the TDS detection device both are connected with a display device, the wastewater pipe is also provided with a wastewater proportioner, the water storage tank and water pipes and the like are made of sterilized materials. Beneficial effects of timely monitoring of a filter assembly, improvement of RO reverse osmosis membrane service life, and reduction of energy consumption and wastewater output volume can be achieved.
Owner:江志鑫
Osmosis water purifier with real-time monitoring
InactiveCN105540889AReal-time monitoring of usage statusExtended service lifeReverse osmosisMultistage water/sewage treatmentFiltrationReverse osmosis
The present invention relates to an osmosis water purifier with real-time monitoring. The osmosis water purifier comprises a water pump, a filtration unit, a RO reverse osmosis membrane treatment unit, and a water storage barrel connected to the purified water outlet pipe of the RO reverse osmosis membrane treatment unit, wherein the filtration unit and the RO reverse osmosis membrane treatment unit are sequentially connected to a water inlet pipe, the water inlet pipe position of the RO reverse osmosis membrane treatment unit is provided with an electromagnetic valve, the filtration unit sequentially comprises a PP filtration core, a granular active carbon filtration core and a compressed carbon filtration core, the water outlet ends of every stage of the filtration cores of the filtration unit are respectively provided with a water flow rate monitoring device, the water outlet end of the RO reverse osmosis membrane treatment unit is provided with a TDS detection device, and a water flow rate detection device and the TDS detection device are connected to a display device. According to the present invention, the osmosis water purifier has beneficial effects of timely filtration assembly monitoring, RO reverse osmosis membrane service life prolonging, energy consumption reducing, and wastewater output reducing.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Backwashing osmosis water purifier capable of sterilizing and intelligent monitoring
InactiveCN105621662AExtended service lifeReduce outputBiocideReverse osmosisTotal dissolved solidsReverse osmosis
The invention relates to a backwashing osmosis water purifier capable of sterilizing and intelligent monitoring. According to the invention, a backwashing unit and a water flow monitoring unit are arranged; the water outlet end of a reverse osmosis (RO) membrane processing unit is provided with a total dissolved solid (TDS) detection unit; a water flow sensor and the total dissolved solid (TDS) detection unit are connected with a programmable logic controller (PLC) which connects the water flow sensor and the total dissolved solid (TDS) detection unit to a display unit and a network transmission unit; the programmable logic controller (PLC) transmits processed signals to the display unit for display; the network transmission unit transmits the processed signals to a terminal unit; and a sterilization material is applied in a water storage bucket, a water pipe, etc. The backwashing osmosis water purifier realizes the following beneficial effects: the service life of a reverse osmosis (RO) membrane is improved; and energy consumption and wastewater output are reduced.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Backwashing bactericidal osmosis water purifier with intelligent monitoring and timing functions
InactiveCN105776646AReal-time monitoring of usage statusExtended service lifeBiocideReverse osmosisWater storageIntelligent lighting
The invention relates to a backwashing bactericidal osmosis water purifier with intelligent monitoring and timing functions. A backwashing wastewater pipe is arranged on the water inlet end of a RO reverse osmosis membrane processing unit, the water outlet pipe of the RO reverse osmosis membrane processing unit is connected to a pressure tank, a non-return valve is arranged on the pipeline that connects the water outlet pipe of the RO reverse osmosis membrane processing unit and a water storage barrel; a timing device (14) is arranged on the water outlet pipe of the RO reverse osmosis membrane processing unit, the timing device (14) is connected to a PLC (15), the PLC (15) starts or closes a water pump according to the signals from the timing device (14); and the water storage barrel and the water pipes all comprise bactericidal materials. The water purifier has the advantages that the service life of RO reverse osmosis membrane is prolonged, and the energy consumption and wastewater output are reduced.
Owner:江志鑫
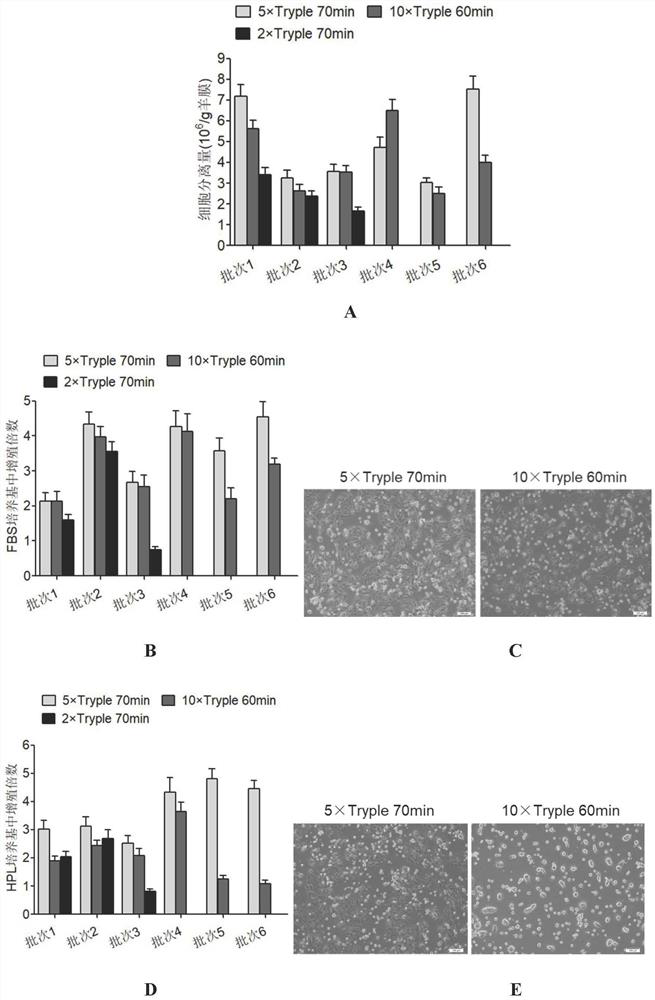
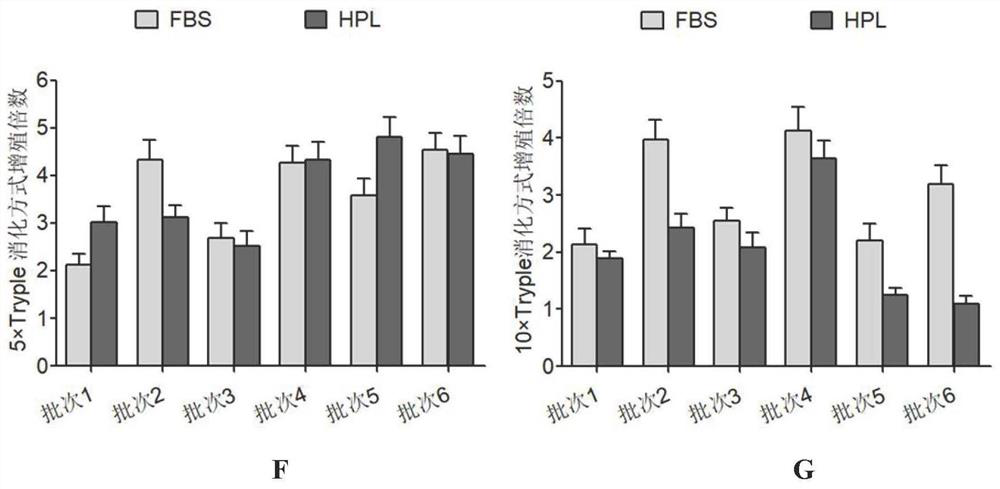
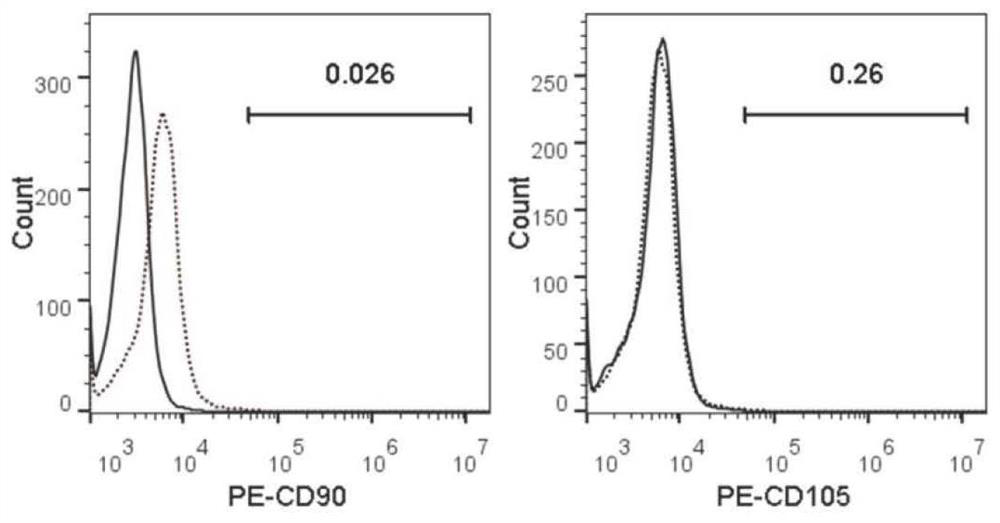
Separation and preparation methods of human amniotic epithelial stem cells
InactiveCN111793596AHigh activityReduce pollutionCell dissociation methodsCulture processSerum freeCulture mediums
The invention discloses separation and preparation methods of human amniotic epithelial stem cells (hAESCs) and the hAESCs. The separation method comprises the following steps of (1) pretreating amniotic tissue with recombinant trypsin, and removing the recombinant trypsin to obtain pretreated amniotic tissue; (2) digesting the pretreated amniotic tissue obtained in the step (1) by using the recombinant trypsin, and collecting digestive juice; (3) adding a digestive terminator into the digestive juice obtained in the step (2), performing filtration and centrifugation on the obtained digestivejuice, discarding a supernatant, and collecting precipitated cells; and (4) inoculating the cells obtained in the step (3) into a serum-free and animal-source-free culture medium system, and performing culture and amplification to obtain the high-purity hAESCs. The hAESCs have the advantages of being rapid to separate, easy and convenient to operate, low in cost and the like, and the obtained hAESCs are directly used for scientific research and clinical treatment and have wide application prospects.
Owner:SHANGHAI ICELL BIOTECH +1
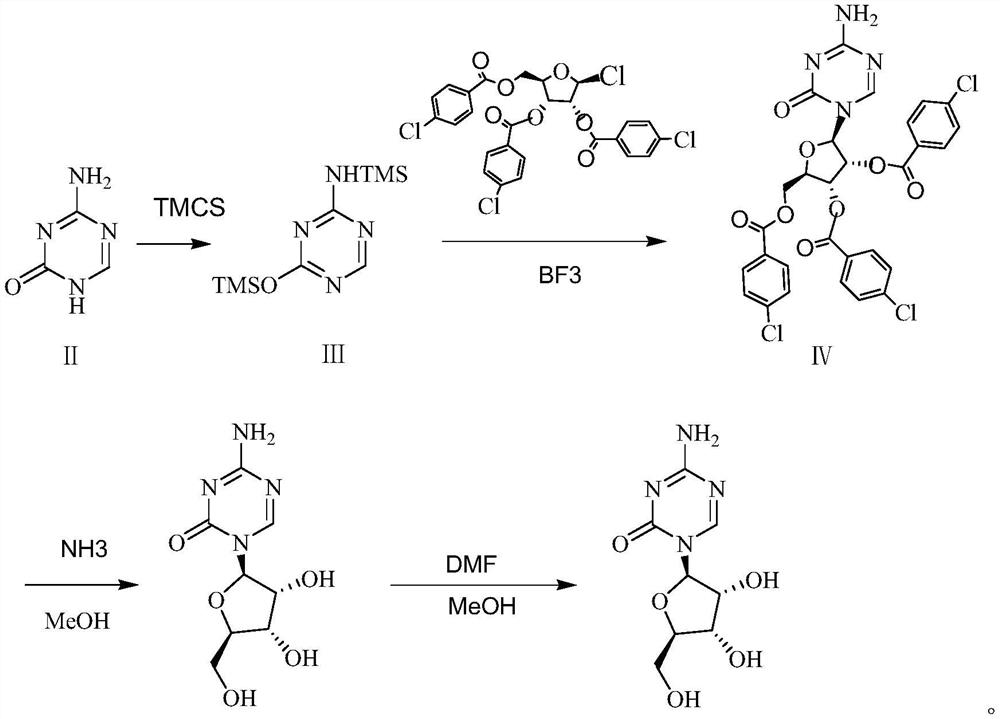
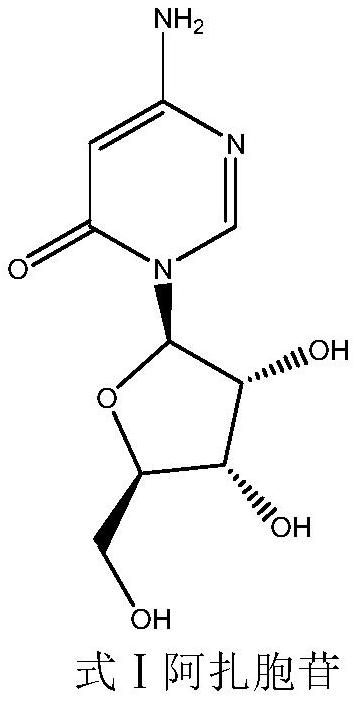
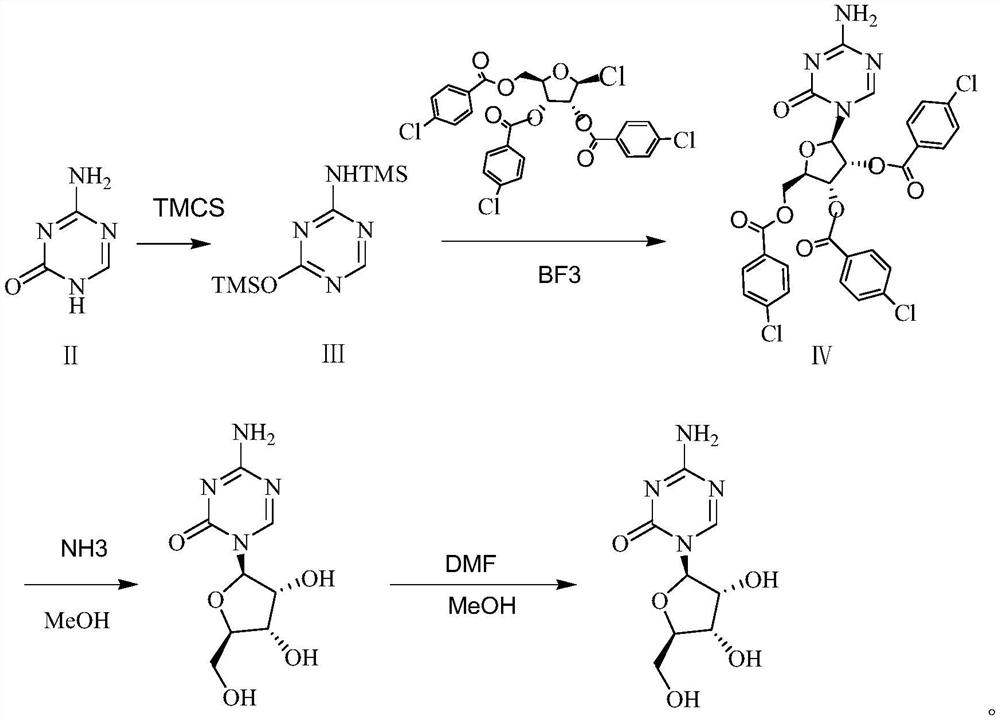
A kind of preparation method of azacitidine
ActiveCN110128485BMild reaction conditionsShort reaction timeSugar derivativesSugar derivatives preparationTrimethylsilyl chloridePhenacyl
The invention belongs to the field of pharmaceutical synthesis, and specifically relates to a preparation method of azacitidine, comprising the following steps: reacting 5-azacytosine and trimethylchlorosilane at 70-80°C for 2 hours to obtain azacitidine Glycoside intermediate Ⅰ; Azacitidine intermediate Ⅰ is dissolved in dichloromethane, under the catalysis of boron trifluoride, and 1-chloro-2,3,5-tri-O-p-chlorobenzoyl-β- D-ribose undergoes a condensation reaction. After the reaction is completed, wash, dry, and suction filter, and the filtrate is distilled under reduced pressure to obtain the azacitidine intermediate II of the formula IV; the azacitidine intermediate II is alcoholylated with ammonia to obtain azacitidine The crude product was purified to obtain high-purity azacitidine. The invention has mild reaction conditions, short reaction time and high yield, and is suitable for industrial production.
Owner:LUNAN BETTER PHARMA
Timed backwash osmotic water purification machine with intelligent monitoring function
InactiveCN105621646AExtended service lifeReduce outputReverse osmosisMultistage water/sewage treatmentWater storage tankWastewater
The invention relates to a timed backwash osmotic water purification machine with an intelligent monitoring function. According to the invention, a backwashing wastewater pipe is arranged at a water inlet end of an RO reverse osmosis membrane treatment unit; a pressure tank is connected to a water outlet pipe of the RO reverse osmosis membrane treatment unit; a check valve is connected to a pipeline connecting the water outlet pipe of the RO reverse osmosis membrane treatment unit and a water storage tank; a timing device 14 is arranged at the water outlet pipe of the RO reverse osmosis membrane treatment unit; the timing device 14 is connected with a PLC 15; and the PLC 15 controls the opening or closing of a water pump according to signals from the timing device 14. With the water purification machine, the beneficial effects that the service life of the RO reverse osmosis membrane is prolonged, energy consumption is reduced, and wastewater output is reduced are achieved.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
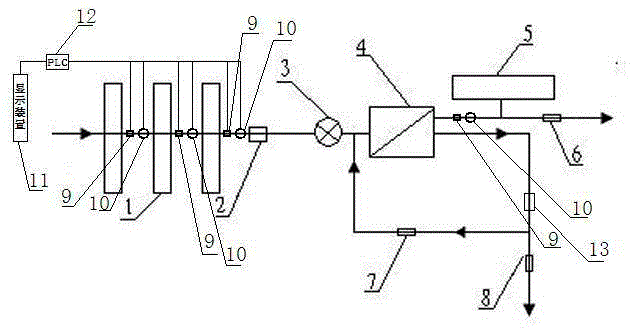
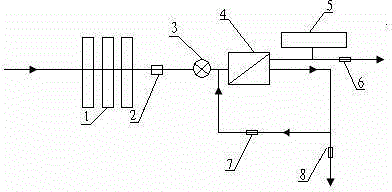
Back-washing sterilizing osmosis water purifier with monitoring
The invention relates to a back-washing sterilizing osmosis water purifier with monitoring. A water outlet pipe of a reverse osmosis (RO) membrane treatment unit is connected to a pressure pot and a one-way valve. A water flow quantity monitoring apparatus and a TDS detection apparatus are arranged on a water outlet end of each stage of filter core in a filtering unit. A waste water pipe is provided with a wastewater proportioner. The water flow quantity monitoring apparatus and the TDS detection apparatus are both connected to a PLC and then are connected to a display apparatus through the PLC. The PLC sends a treated signal to the display apparatus. A water pump is connected to the PLC which controls on / off of the water pump. A water storage barrel and water pipes are all made from a sterilizing material. The service life of the RO membrane is prolonged, so that energy consumption and wastewater production amount are reduced.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
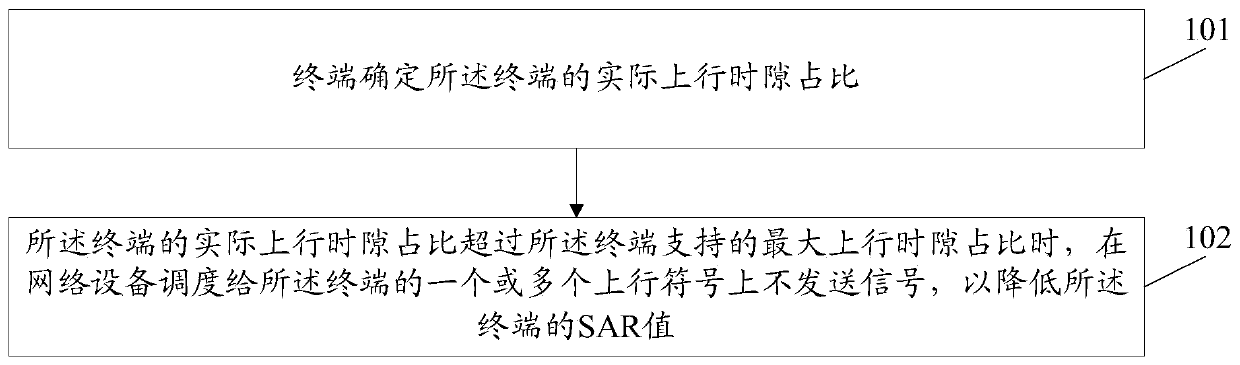
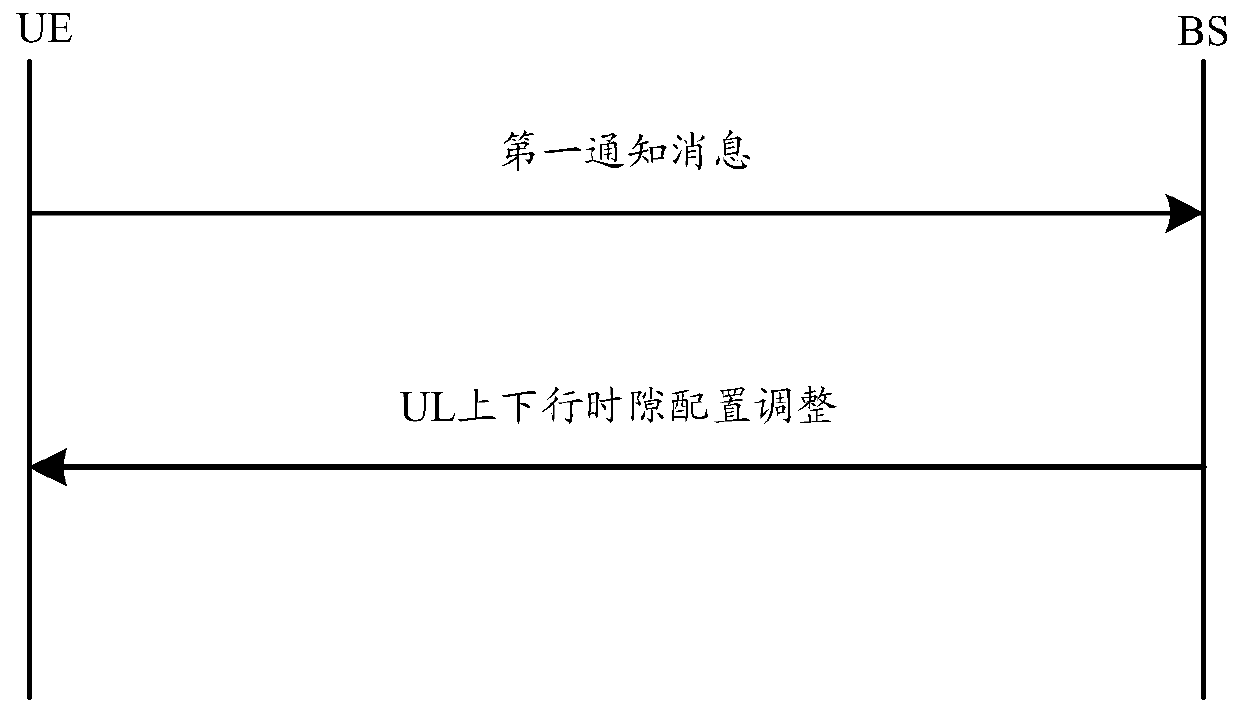
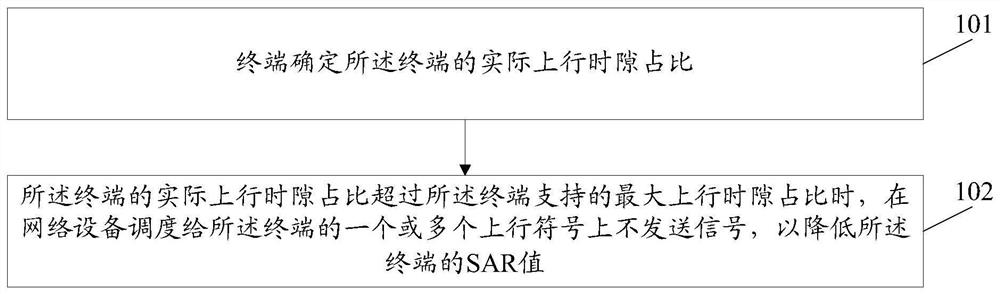
Method and device for reducing SAR value and computer storage medium
ActiveCN110493871AAvoid over-standard problemsThe proportion of uplink time slots is reducedWireless communicationSynthetic aperture radarComputer terminal
The invention discloses a method and a device for reducing an SAR (Synthetic Aperture Radar) value and a computer storage medium. The method comprises the following steps: a terminal determines an actual uplink time slot proportion of the terminal; and when the actual uplink time slot ratio of the terminal exceeds the maximum uplink time slot ratio supported by the terminal, a signal is not sent on one or more uplink symbols scheduled to the terminal by network equipment so as to reduce the SAR value of the terminal.
Owner:GUANGDONG OPPO MOBILE TELECOMM CORP LTD
Sterilization osmotic water purification machine with monitoring function
InactiveCN105621694AExtended service lifeAvoid breedingBiocideReverse osmosisSolenoid valveWater storage tank
The invention relates to a sterilization osmotic water purification machine with a monitoring function. According to the invention, a wastewater outlet end of an RO reverse osmosis membrane treatment unit is connected with a wastewater pipe; the wastewater pipe discharges wastewater; a clean water outlet end is connected to a water storage tank. The water storage tank is connected to the clean water outlet end. A solenoid valve is connected at a water inlet pipe of the RO reverse osmosis membrane treatment unit; a water flow monitoring device is arranged at the water outlet end of each level of filter core of a filtering unit; TDS detection devices are arranged at the water outlet end of the RO reverse osmosis membrane treatment unit; the water flow monitoring devices and the TDS detection devices are all connected with a display device; the wastewater pipe is also provided with a wastewater proportioner; and sterilization materials are adopted to manufacture the water storage tank, water pipes and the like. With the water purification machine, the filtering assembly can be monitored in real time, the service life of the RO reverse osmosis membrane is prolonged, energy consumption is reduced, and wastewater output is reduced.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
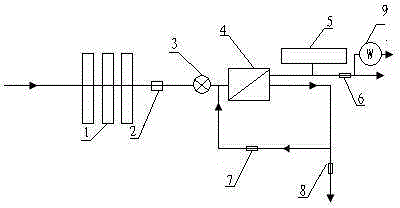
Combined reverse-osmosis (RO) constant-pressure water purifier with heating function
InactiveCN104671529AExtended service lifeAccurately grasp the replacement timeMultistage water/sewage treatmentWater storageWastewater
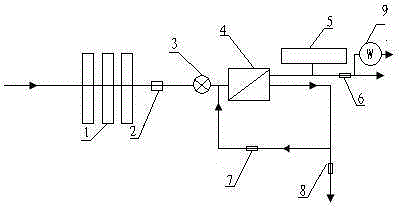
The invention relates to a water purifier with a heating function, particularly relates to a combined reverse-osmosis (RO) constant-pressure water purifier with a heating function, and aims at prolonging the service life of an RO membrane, increasing the water purifying efficiency, accurately mastering the replacing time of the RO membrane and effectively avoiding the problem that bacteria in purified water exceed the standard. In order to achieve the aims, the invention provides the technical scheme as follows: the combined RO constant-pressure water purifier with the heating function comprises a water pump, a filtering unit, an RO membrane treatment unit and a water storage barrel, wherein the filtering unit and the RO membrane treatment unit are sequentially connected with a water inlet pipe; the water storage barrel is connected with a purified water outlet pipe of the RO membrane treatment unit; a water inlet pipe of the RO membrane treatment unit is provided with an electromagnetic valve; a wastewater pipe of the RO membrane treatment unit is also connected with a water return pipe for returning wastewater to the water inlet end of the RO membrane treatment unit; and the end of the purified water outlet pipe is also connected with a heating tank. The combined RO constant-pressure water purifier with the heating function has the beneficial effects of prolonging the service life of the RO membrane, effectively inhibiting bacteria and reducing the energy consumption and wastewater yield.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
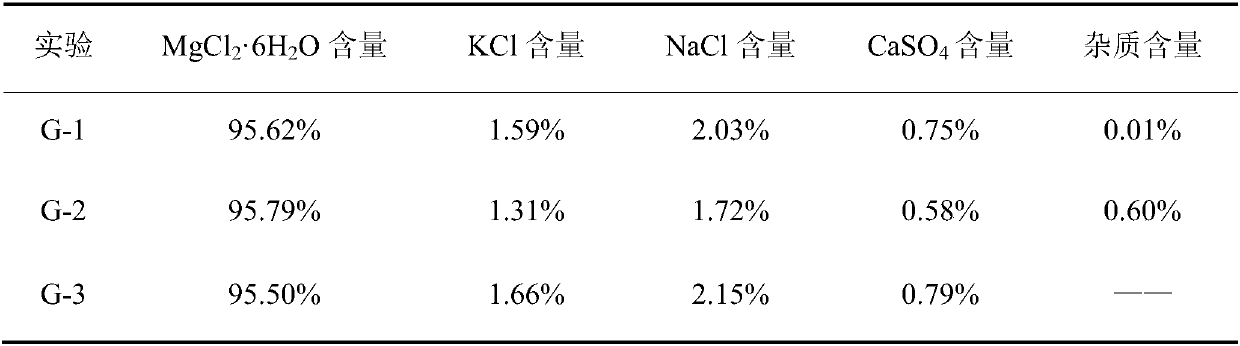
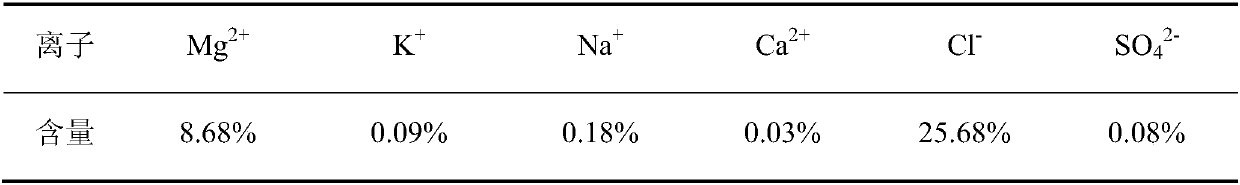
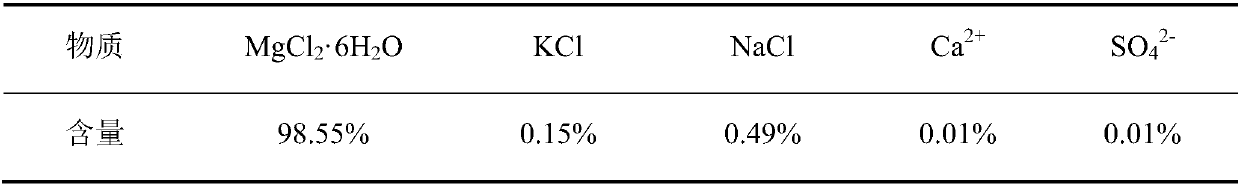
The preparation method of edible magnesium chloride
ActiveCN106044807BAvoid over-standard problemsReduce contentMagnesium chloridesCalcium/strontium/barium sulfatesLoss rateFiltration
A preparing method of edible magnesium chloride is disclosed. The method includes steps of A) preparing a first mother liquor, B) subjecting the first mother liquor to first evaporation at 100-110 DGE C, and performing hot filtration to obtain a filtrate that is adopted as a second mother liquor, with the water loss rate of the first mother liquor in the first evaporation being 4.1-16.3%, and C) subjecting the second mother liquor to second evaporation, cooling until the temperature is lower than 90 DEG C, and performing solid liquid separation to obtain a solid phase that is the edible magnesium chloride, wherein the first mother liquor comprises Na<+>, K<+>, Mg<2+>, Cl<->, SO4<2-> and H2O, and the first mother liquor comprises 0.1-0.2% by mass of the Na<+>, 0.05-0.1% by mass of the K<+>, 8.4-9.5% by mass of the Mg<2+>, 24.5-28% by mass of the Cl<-> and 0.05-0.1% by mass of the SO4<2->, with the balance being the H2O. The method is simple and avoids a problem that mother liquor entrainment exceeds standards due to one-time evaporation.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Backwashing bactericidal osmosis water purifier with monitoring function
InactiveCN105776638AReduce outputIncrease productivityMultistage water/sewage treatmentWater storageWastewater
The invention relates to a backwashing bactericidal osmosis water purifier with a monitoring function. A backwashing wastewater pipe is arranged on the water inlet end of a RO reverse osmosis membrane processing unit, the water outlet pipe of the RO reverse osmosis membrane processing unit is connected to a pressure tank, a non-return valve is arranged on the pipeline that connects the water outlet pipe of the RO reverse osmosis membrane processing unit and a water storage barrel, and the water storage barrel and water pipes all comprise bactericidal materials. The water purifier has the advantages that the service life of RO reverse osmosis membrane is prolonged, the energy consumption is reduced, and the wastewater yield is reduced.
Owner:江志鑫
A method and device for reducing SAR value, and computer storage medium
ActiveCN110493871BReduce SAR valueAvoid over-standard problemsWireless communicationSignal onComputer engineering
Owner:GUANGDONG OPPO MOBILE TELECOMM CORP LTD
Backwashing osmosis water purifier capable of intelligent monitoring and timing
InactiveCN105621717AReal-time monitoring of usage statusExtended service lifeSemi-permeable membranesWater/sewage treatment bu osmosis/dialysisIntelligent lightingWater storage
The invention relates to a backwashing osmosis water purifier capable of intelligent monitoring and timing. According to the invention, the water inlet end of a reverse osmosis (RO) membrane processing unit is provided with a backwashing wastewater pipe; a water outlet pipe of the reverse osmosis (RO) membrane processing unit is connected with a pressure tank; a pipeline connecting the reverse osmosis (RO) membrane processing unit to a water storage bucket is also connected with a one-way valve; the water outlet pipe of the reverse osmosis (RO) membrane processing unit is provided with a timing unit 14; the timing unit 14 and a programmable logic controller (PLC) 15 are in connection; and the programmable logic controller (PLC) 15 controls opening or closing of a water pump according to signals of the timing unit 14. The backwashing osmosis water purifier realizes the following beneficial effects: the service life of a reverse osmosis (RO) membrane is improved; and energy consumption and wastewater output are reduced.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Combined reverse-osmosis (RO) water purifier with heating function
InactiveCN104671532AReduce outputIncrease productivityWater/sewage treatment bu osmosis/dialysisMultistage water/sewage treatmentWater storageWastewater
The invention relates to a water purifier, particularly relates to a combined reverse-osmosis (RO) water purifier with a heating function, and aims at prolonging the service life of an RO membrane, increasing the water purifying efficiency, accurately mastering the replacing time of the RO membrane and effectively avoiding the problem that bacteria in purified water exceed the standard. In order to achieve the aims, the invention provides the technical scheme as follows: the combined RO water purifier with the heating function comprises a water pump, a filtering unit, an RO membrane treatment unit and a water storage barrel, wherein the filtering unit and the RO membrane treatment unit are sequentially connected with a water inlet pipe; the water storage barrel is connected with a purified water outlet pipe of the RO membrane treatment unit; a water inlet pipe of the RO membrane treatment unit is provided with an electromagnetic valve; a wastewater pipe of the RO membrane treatment unit is also connected with a water return pipe for returning wastewater to the water inlet end of the RO membrane treatment unit; and the end of the purified water outlet pipe is also connected with a heating tank. The combined RO water purifier with the heating function has the beneficial effects of prolonging the service life of the RO membrane and reducing the energy consumption and wastewater yield.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Backwash sterilization osmotic water purification machine with monitoring function
InactiveCN105621687AReduce outputIncrease productivityBiocideReverse osmosisWater storage tankWastewater
The invention relates to a backwash sterilization osmotic water purification machine with a monitoring function. According to the invention, a backwashing wastewater pipe is arranged at a water inlet end of an RO reverse osmosis membrane treatment unit; a pressure tank is connected at the water outlet pipe of the RO reverse osmosis membrane treatment unit; a check valve is arranged on a pipeline connecting the water outlet pipe of the RO reverse osmosis membrane treatment unit and a water storage tank; and sterilization materials are adopted to manufacture the water storage tank, water pipes and the like. With the water purification machine, the service life of the RO reverse osmosis membrane is prolonged, energy consumption is reduced, and wastewater output is reduced.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Sterilization backwash osmotic water purification machine with monitoring function
The invention relates to a sterilization backwash osmotic water purification machine with a monitoring function. According to the invention, a pressure tank and a check valve are connected to the water outlet pipe of an RO reverse osmosis membrane treatment unit; a water flow monitoring device and a TDS detection device are arranged at the water outlet end of each level of filter core of each filtering unit; a wastewater pipe is provided with a wastewater proportioner; the water flow monitoring devices and the TDS detection devices are all connected with a PLC, and are connected with a display device through the PLC; The PLC transmits processed signals to the display device; a water pump is connected to the PLC; the opening or closing of the water pump is controlled by the PLC; and sterilization materials are adopted to manufacture a water storage tank, water pipes and the like. With the water purification machine, the beneficial effects that the service life of the RO reverse osmosis membrane is prolonged, energy consumption is reduced, and wastewater output is reduced are achieved.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Harmless treatment method for barium residues and arsenic residues
The invention provides a harmless treatment method for barium residues and arsenic residues. The harmless treatment method comprises the steps that the barium residues, the arsenic residues and a stabilized agentia are mixed to obtain a waste residue mixture for piling and maintaining. According to the harmless treatment method, the barium residues and the arsenic residues are subjected to harmless treatment at the same time, the characters of the two kinds of hazardous waste are fully utilized, soluble barium in the barium residues and soluble arsenic in the arsenic residues react with each other, indissolvable arsenic acid is generated, and therefore the aim of stabilizing the barium residues and the arsenic residues is achieved, and the effect of treating waste with waste is achieved; and the agentia dosage and capacity increase in the harmless treatment process of the barium residues and the arsenic residues can be reduced, the cost of the harmless treatment is greatly reduced, other environment problems caused by the large use amount of the stabilizing agentia are avoided, and the cost advantage of treating the barium residues and the arsenic residues with high leaching concentration is more obvious.
Owner:中化环境修复(上海)有限公司 +1
Sterilization backwash osmotic water purification machine with monitoring function
InactiveCN105621707AReal-time monitoring of usage statusExtended service lifeBiocideReverse osmosisWater storage tankReverse osmosis
The invention relates to a sterilization backwash osmotic water purification machine with monitoring function. According to the invention, a backwashing wastewater pipe is arranged at a water inlet end of an RO reverse osmosis membrane treatment unit; a pressure tank is connected at a water outlet pipe of the RO reverse osmosis membrane treatment unit; a check valve is connected to a pipeline connecting the outlet pipe of the RO reverse osmosis membrane treatment unit and a water storage tank; and sterilization materials are adopted to manufacture a water storage tank, water pipes and the like. With the water purification machine, RO reverse osmosis membrane service life can be prolonged, energy consumption can be reduced, and wastewater output can be reduced.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Sterilization water purification machine with monitoring function
InactiveCN105621696AExtended service lifeAvoid breedingBiocideReverse osmosisSolenoid valveWater storage tank
The invention relates to a sterilization water purification machine with a monitoring function. According to the invention, a wastewater outlet end of an RO reverse osmosis membrane treatment unit is connected with a wastewater pipe; the wastewater pipe discharges wastewater; a purified water outlet end is connected to a water storage tank; the water storage tank is connected to the purified water outlet end; a solenoid valve is arranged at a water inlet pipe of the RO reverse osmosis membrane treatment unit; a water flow monitoring device is arranged at the water outlet end of each level of filter core of each filtering unit; TDS detection devices are arranged at the water outlet end of the RO reverse osmosis membrane treatment unit; the water flow monitoring devices and the TDS detection devices are all connected with a display device; the wastewater pipe is also provided with a wastewater proportioner; and sterilization materials are adopted to manufacture the water storage tank, water pipes and the like. With the water purification machine, the beneficial effects that the filtering assembly can be monitored in time, the service life of the RO reverse osmosis membrane is prolonged, energy consumption is reduced, and wastewater output is reduced are achieved.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Backwash sterilization water purification machine with monitoring function
InactiveCN105621688AExtended service lifeReduce outputBiocideReverse osmosisWater storage tankReverse osmosis
The invention relates to a backwash sterilization water purification machine with monitoring function. A pressure tank and a check valve are connected at a water outlet pipe of an RO reverse osmosis membrane treatment unit; a water flow monitoring device and a TDS detection device are arranged at a water outlet end of each level of filtering core of a filtering unit; a wastewater proportioner is arranged on the wastewater pipe. The water flow monitoring devices and the TDS detection devices are connected with a PLC; the PLC is connected with a display device; the PLC transmits processed signals to the display device; a water pump is connected to the PLC; the PLC controls the on / off of the water pump; and sterilization materials are adopted to manufacture the water storage tank, water pipes and the like. With the water purification machine, the service life of the RO reverse osmosis membrane is prolonged, energy consumption is reduced, and wastewater output is reduced.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Backwashing osmosis water purifier with bactericidal and monitoring functions
InactiveCN105776641AAccurately grasp the replacement timeAvoid over-standard problemsMultistage water/sewage treatmentWater storageWater discharge
The invention relates to a backwashing osmosis water purifier with bactericidal and monitoring functions. The water discharge pipe of a RO reverse osmosis membrane processing unit is connected to a pressure tank and a non-return valve; the water discharge ends of each filter core of a filter unit are all provided with a water flow monitoring device and a TDS detection device; the wastewater pipe is provided with a wastewater ratio device; the water flow detection devices and the TDS detection devices are all connected to a PLC and then connected to a display device through the PLC; the processed signals are transmitted to the display device by the PLC, a water pump is connected to the PLC, the water pump is started or closed by the PLC, and the water storage barrel and water pipes all comprise bactericidal materials. The water purifier has the advantages that the service life of RO reverse osmosis membrane is prolonged, the energy consumption is reduced, and the wastewater yield is reduced.
Owner:江志鑫
A combined type reverse-osmosis bacteriostatic water purifier
InactiveCN104671466AExtended service lifeAccurately grasp the replacement timeWater/sewage treatment bu osmosis/dialysisReverse osmosisWater storageWater discharge
The invention relates to a water purifier and particularly relates to a combined type reverse-osmosis bacteriostatic water purifier. The technical problems to be overcome are to prolong the service lifetime of RO (reverse osmosis) membranes, to increase the water purification efficiency, to accurately master the replacing time of the RO (reverse osmosis) membrane, and to effectively avoid a circumstance that the bacteria amount of purified water exceeds a threshold. To achieve the objectives, a technical scheme as follows is provided: the water purifier comprises a water pump, a filtering unit, an RO (reverse osmosis) membrane treatment unit and a water storage barrel connected to a purified water discharging pipe of the RO (reverse osmosis) membrane treatment unit, wherein the filtering unit and the RO (reverse osmosis) membrane treatment unit are connected to a water feeding pipe in order; the water feeding pipe of the RO (reverse osmosis) membrane treatment unit is provided with a magnetic valve; a waste water pipe of the RO (reverse osmosis) membrane treatment unit is provided with a water return pipe; and the water return pipe is used for returning waste water to a water feeding end of the RO (reverse osmosis) membrane treatment unit. The water purifier achieves beneficial effects of prolonging the service lifetime of RO (reverse osmosis) membranes, achieving effective bacteriostasis, and reducing energy consumption and the output of waste water.
Owner:TAOEE QINGDAO WATER PURIFICATION EQUIP MFG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com