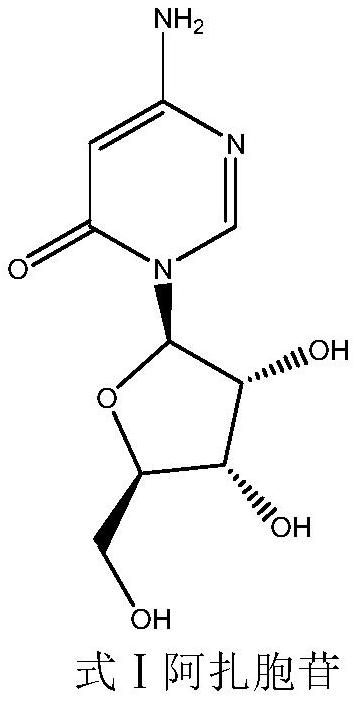
A kind of preparation method of azacitidine
A technology for azacitidine and azacytosine, which is applied in the field of preparation of azacitidine, can solve the problems of increased risk, complicated process, long time consumption, etc., and achieves improved purity and yield, mild reaction conditions, and high reaction efficiency. short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
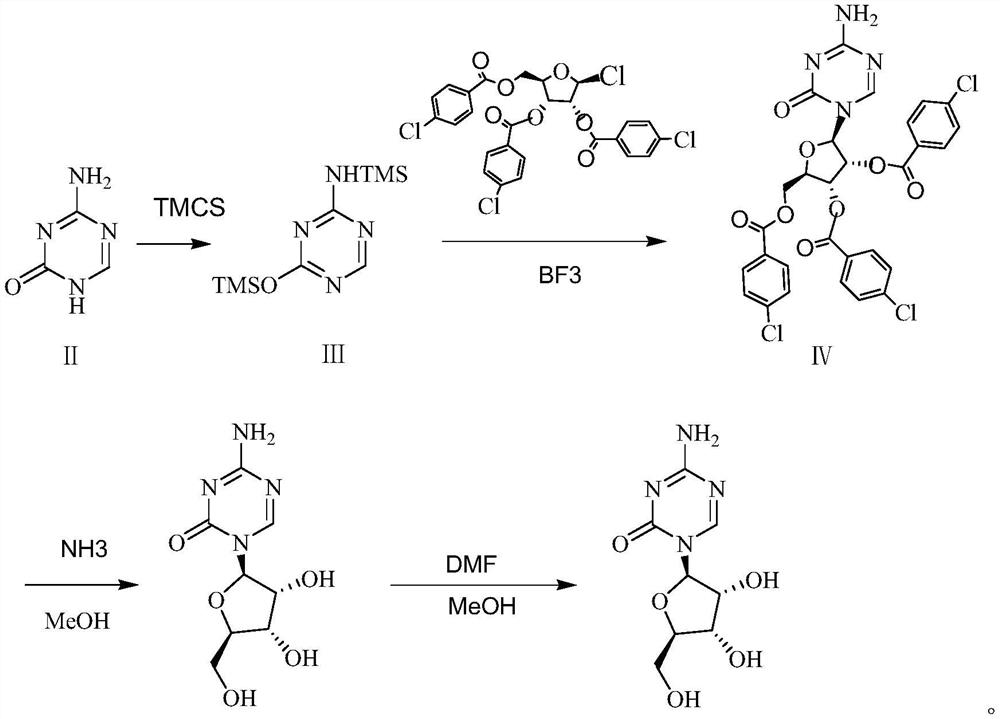
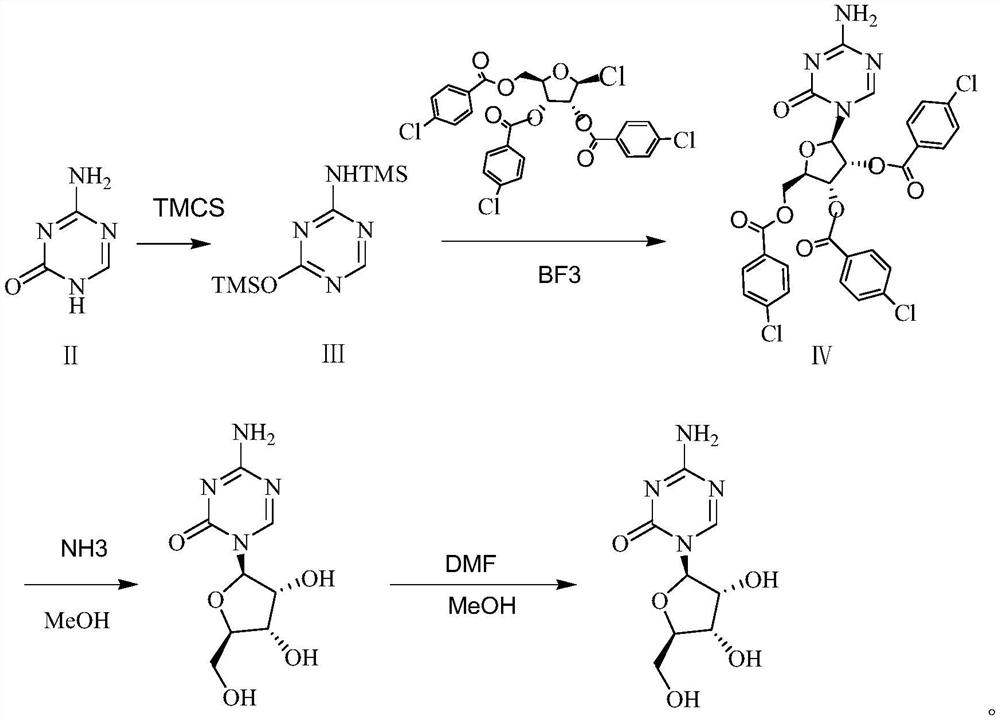
[0025] Put 33.6g of 5-azacytosine into a three-necked flask, add 134.4ml of trimethylchlorosilane and 0.85g of ammonium sulfate, heat up to 70°C, the solution is clarified for about 2h, and then the solvent is evaporated under reduced pressure to constant weight. Zacytidine Intermediate I.
[0026] Dissolve 76.8g (0.3mol) of azacitidine intermediate I with 614.4ml of dichloromethane, put it into a three-necked flask and stir, and then add 0.27mol of 1-chloro-2,3,5-tri-O-p-chlorobenzyl Acyl-β-D-ribose, slowly add 0.33mol boron trifluoride dropwise, control the temperature to -5 °C and stir the reaction, after the reaction for 2 hours, add saturated brine, and separate the liquid to obtain the organic phase, and then add saturated hydrogen carbonate to the organic phase. Sodium solution, separated to obtain the organic phase, dried over anhydrous sodium sulfate for 2 hours, suction filtered to obtain the organic phase, and distilled under reduced pressure to constant weight to o...
Embodiment 2
[0029] Put 33.6g of 5-azacytosine into a three-necked flask, add 336ml of trimethylchlorosilane and 0.85g of ammonium sulfate, heat up to 80°C, the solution is clarified for about 2h, and then the solvent is evaporated under reduced pressure to a constant weight to obtain a Zacytidine Intermediate I.
[0030] Dissolve 76.8g (0.3mol) of azacitidine intermediate I with 1536ml of dichloromethane, put it into a three-necked flask and stir, and then add 0.39mol of 1-chloro-2,3,5-tri-O-p-chlorobenzoyl -β-D-ribose, slowly add 0.45mol boron trifluoride dropwise, control the temperature to 15 °C and stir the reaction, after the reaction for 2 hours, add saturated brine, and separate the liquid to obtain the organic phase, and then add saturated sodium bicarbonate solution to the organic phase. , the organic phase was obtained by liquid separation, dried over anhydrous sodium sulfate for 2 hours, and the organic phase was obtained by suction filtration, which was distilled under reduced...
Embodiment 3
[0033] Put 33.6g of 5-azacytosine into a three-necked flask, add 168ml of trimethylchlorosilane and 0.85g of ammonium sulfate, heat up to 80°C, the solution is clarified for about 2 hours, and then the solvent is evaporated under reduced pressure to constant weight to obtain aza Cytidine Intermediate I.
[0034] Dissolve 76.8g (0.3mol) of azacitidine intermediate I with 691.2ml of dichloromethane, put it into a three-necked flask and stir, and then add 0.285mol of 1-chloro-2,3,5-tri-O-p-chlorobenzyl Acyl-β-D-ribose, slowly add 0.36mol boron trifluoride dropwise, control the temperature to 5 °C and stir the reaction, after 2 hours of reaction, add saturated brine, and separate the liquid to obtain an organic phase, and then add saturated sodium bicarbonate to the organic phase. The solution was separated and the organic phase was obtained. After drying with anhydrous sodium sulfate for 2 hours, the organic phase was obtained by suction filtration, and the azacitidine intermedia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com