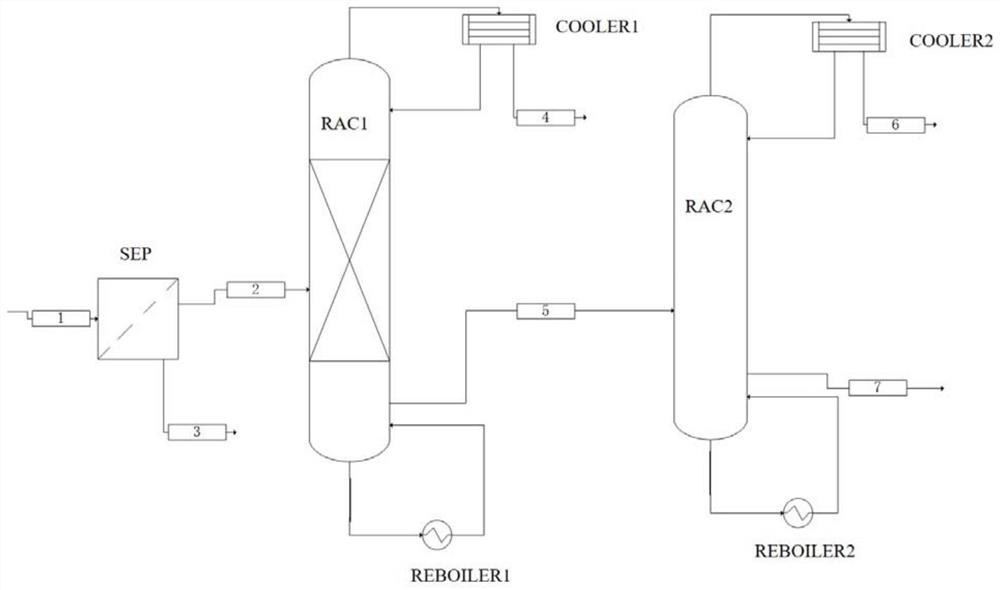
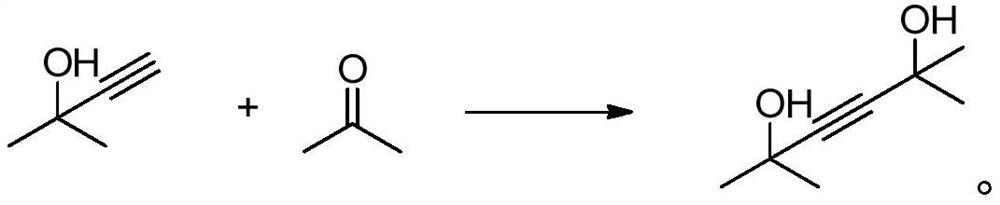
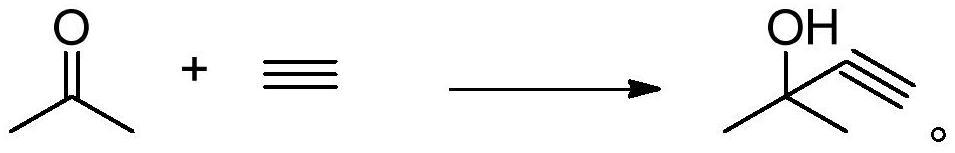Separation method of 2-methyl-3-butyn-2-ol
A separation method and technology of butyne, applied in the separation/purification of hydroxy compounds, chemical instruments and methods, preparation of hydroxy compounds, etc., can solve the problems of product conversion rate and selectivity reduction, high content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: the preparation of 1# reaction solution
[0083] First, replace the 500L autoclave with ammonia gas three times, lower the temperature in the reactor to -20°C, add liquid ammonia (144.5Kg, 8500mol), start stirring, and feed acetylene (16800L, 750mol) at a concentration of 20wt%. Magnesium sulfate aqueous solution 43.5g and concentration are 50wt% potassium hydroxide aqueous solution (1008g, the potassium hydroxide consumption as solute is 3mol% of acetone), be warming up to 10 ℃ and add acetone (17.4Kg, 300mol), control acetone to add The feed rate is completed in about 1h; the reaction temperature is 10°C, and after 2h of reaction, 5Kg of ammonium sulfate aqueous solution with a concentration of 10wt% is added for neutralization to obtain 1# reaction containing 2-methyl-3-butyn-2-ol solution, and sampled to detect the reaction solution by GC.
[0084] The composition of 1# reaction solution: the content of inorganic salt is 2.16wt%, the content of water ...
Embodiment 2
[0085] Embodiment 2: the preparation of 2# reaction solution
[0086] First, replace the 500L autoclave with ammonia gas three times, lower the temperature in the reactor to -20°C, add liquid ammonia (144.5Kg, 8500mol), start stirring, and feed acetylene (16800L, 750mol) at a concentration of 20wt%. Zinc acetate aqueous solution 17.5g, then add concentration and be the potassium hydroxide aqueous solution (1008g of 50wt%, the potassium hydroxide consumption as solute is 3mol% of acetone), be warming up to 10 ℃ and add acetone (17.4Kg, 300mol), control The acetone addition rate is about 1h and the feed is completed; the reaction temperature is 10°C, and after 2 hours of reaction, 5Kg of ammonium sulfate aqueous solution with a concentration of 10wt% is added for neutralization to obtain 2-methyl-3-butyn-2-ol containing 2 #Reaction solution, and take a sample to detect the reaction solution by GC.
[0087] The composition of 2# reaction solution: the content of inorganic salt i...
Embodiment 3
[0088] Embodiment 3: the preparation of 3# reaction solution
[0089] First, replace the 500L autoclave with ammonia gas three times, lower the temperature in the reactor to -20°C, add liquid ammonia (144.5Kg, 8500mol), start stirring, and feed acetylene (16800L, 750mol) at a concentration of 20wt%. Zinc acetate aqueous solution 8.7g, then adding concentration is 50wt% potassium hydroxide aqueous solution (1008g, the potassium hydroxide consumption as solute is 3mol% of acetone), be warming up to 10 ℃ and add acetone (17.4Kg, 300mol), control The acetone addition rate is about 1h, and the feed is completed; the reaction temperature is 10°C, and after 2 hours of reaction, 5Kg of ammonium sulfate aqueous solution with a concentration of 10wt% is added for neutralization to obtain 3-butyn-2-ol containing 2-methyl-3- #Reaction solution, and take a sample to detect the reaction solution by GC.
[0090] The composition of 3# reaction solution: the content of inorganic salt is 2.17w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com