Iridium complex, preparation method thereof and photodynamic therapy drug
A technology of iridium complexes and compounds, applied in the field of biomedicine, can solve the problems of poor water solubility, long cell penetration time, low cell uptake rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
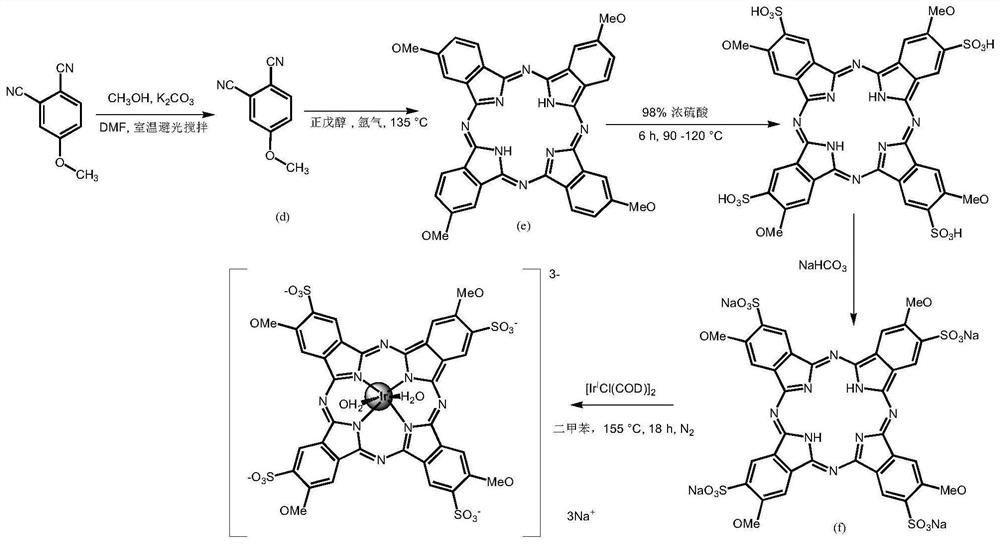
[0061] The second aspect of the embodiment of the present application provides a method for preparing an iridium complex, comprising the following steps:
[0062] S10. Compound A is mixed with concentrated sulfuric acid and subjected to a sulfonation reaction to separate and obtain sulfonated compound B;
[0063] S20. Using the first basic salt to neutralize compound B to obtain compound C;
[0064] S30. Dissolving the compound C and the iridium precursor in the first organic solvent, performing a coordination reaction, and separating to obtain the first iridium complex;
[0065] Wherein, the structural general formula of compound A is:
[0066] or
[0067] When the structural formula of the compound A is A1, the structural formula of the first iridium complex is I-1; when the structural formula of the compound A is A2, the structural formula of the first iridium complex is II-1:
[0068]
[0069] Among them, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 are ...
Embodiment 1
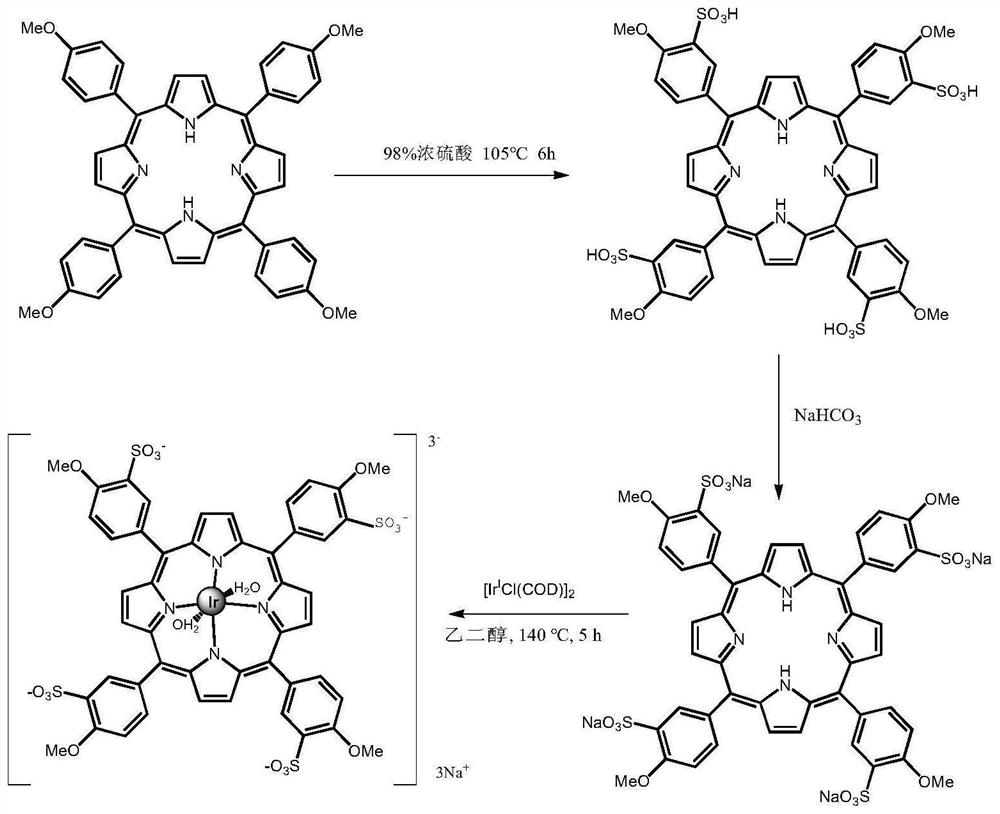
[0138] A kind of methoxy-porphyrin-iridium complex, the synthetic route diagram is attached figure 1 As shown, the following preparation steps are included:
[0139] ①Take 4.00g TMPP (5,10,15,20-tetrakis-(4-methoxyphenyl)-porphyrin) and 40.0mL (98.0%H 2 SO 4 ) in a mortar and grind to a uniform paste. The paste was transferred to a 250 mL round bottom flask, then 60 mL of 98% H 2 SO 4 , and the mixture was stirred and heated in an oil bath at 105° C. for 6 h.
[0140]②Put the mixture in a 400mL beaker and let it stand at room temperature for 48 hours. Then slowly add 300 mL of deionized water and stir. After cooling, filter with a G5 sintered glass filter to remove unreacted TMPP in the solution, add 200 mL of acetone to the filtrate, and stir to precipitate out the precipitate. The resulting precipitate was washed twice with acetone. Acetone was removed, and the solid product was dissolved in 150 mL of deionized water and washed with saturated NaHCO 3 Neutralized to ...
Embodiment 2
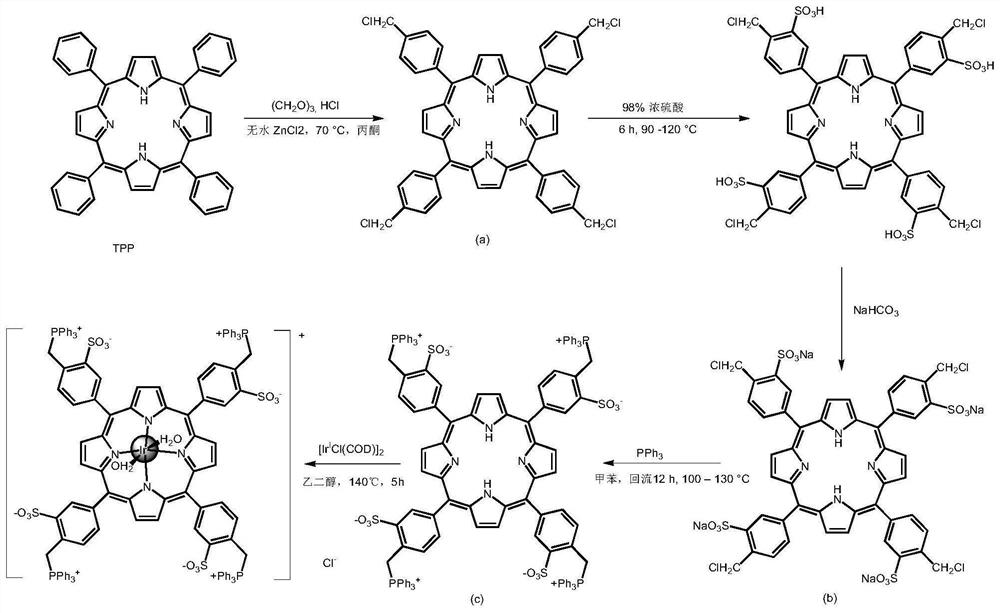
[0144] A kind of triphenylphosphine-porphyrin-iridium complex, the synthetic route diagram is as attached figure 2 As shown, the following preparation steps are included:
[0145] ① Take TPP (tetraphenylporphyrin, 4.00g, 6.5mmol) and paraformaldehyde (0.1351g, 1.5mmol), appropriate amount of hydrochloric acid, anhydrous zinc chloride and acetone, mix them in a 150mL round-bottomed flask, 70 ℃, 3h. After the reaction was finished, it was washed with deionized water, and the organic phase was separated and retained. The organic phase was rotary evaporated to remove acetone, and the solid was separated and purified by column chromatography to obtain compound a.
[0146] ②Take a and 40.0mL (98.0%H 2 SO 4 ) in a mortar and grind to a uniform paste. The paste was transferred to a 250mL round bottom flask, and then 60mL of 98% H 2 SO 4 , and the mixture was stirred and heated in an oil bath at 90-120°C for 6h. Put the mixture in a 400mL beaker, cool to room temperature, then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com