Ruthenium coordination compound with near-infrared fluorescence, preparation method and application thereof
A ruthenium complex, near-infrared technology, applied in the field of medicine, can solve the problems of large toxic and side effects, large surgical trauma, affecting the quality of life of patients, etc., and achieve the effect of great application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
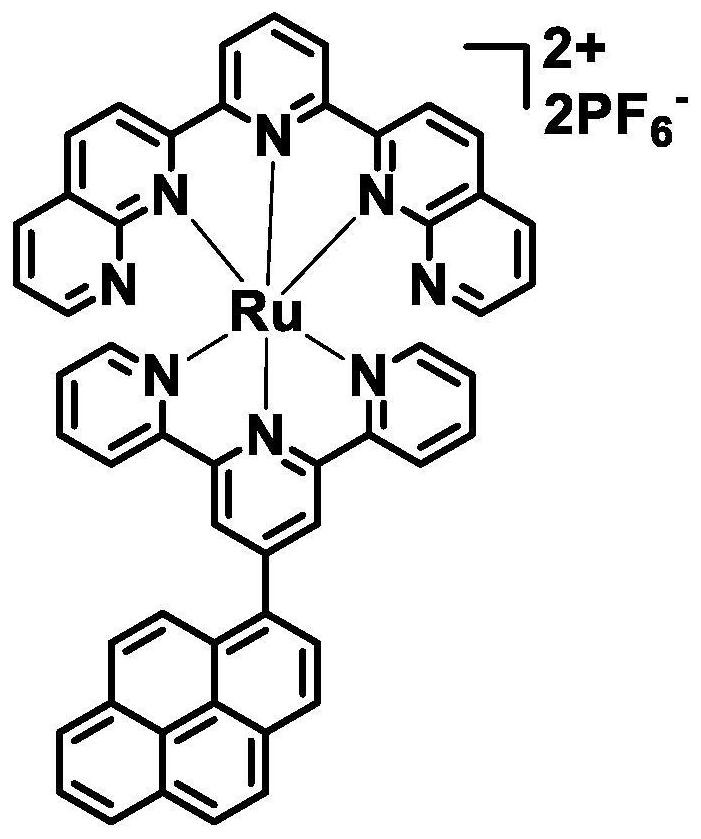
[0046] The structural formula of the ruthenium complex with near-infrared fluorescence is as follows figure 1 shown. Its synthesis method is as follows:
[0047] S1. Formation of bnp ligand by reaction of 2,6-diacetylpyridine with 2-amino-3-pyridinecarbaldehyde
[0048] 2,6-diacetylpyridine (1.6317g, 10mmol) and 2-amino-3-pyridinecarbaldehyde (2.4426g, 20mmol) and sodium hydroxide (0.4g, 10mmol) were heated to 95°C in ethanol (100ml), After 12 hours of reflux reaction, the reaction was cooled to room temperature and the solid was filtered out. The filtered solid was washed with ethanol and dried in vacuum to obtain 3.2886 g of yellow solid bnp with a yield of 98.06%. The above chemical reaction equation is as follows:
[0049]
[0050] Mass spectrum: 336.2, [M+H] + ,358.2,[M+Na] + ;
[0051] H NMR spectrum: 1 H NMR (400MHz, CDCl 3 ):δ9.19(s,2H),9.01(d,J=8.4Hz,4H),8.40(d,J=8.5Hz,2H),8.28(d,J=7.6Hz,2H),8.13(s ,1H), 7.55(s,2H).
[0052] S2. Reaction of 2-acetylpyridi...
Embodiment 2
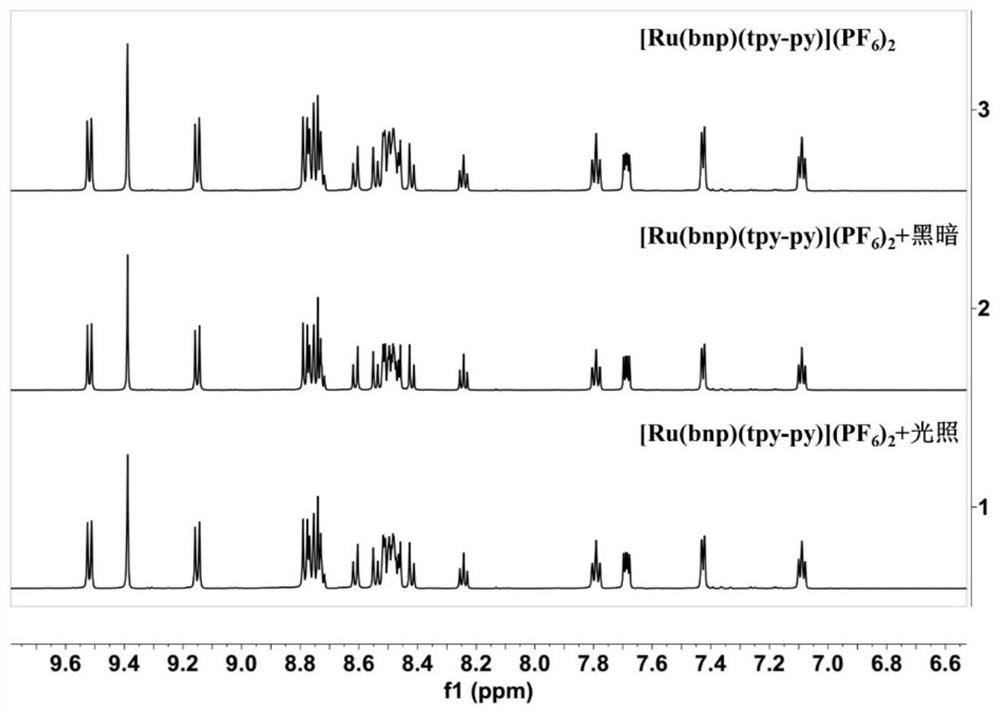
[0066] Dark stability and photostability of the near-infrared fluorescent ruthenium complex obtained in Example 1
[0067] The dark stability and photostability properties of ruthenium complexes were analyzed by H NMR spectroscopy. The ruthenium complex is made into a solution (control sample) with dimethyl sulfoxide-d6 and added to the NMR tube to record its proton NMR spectrum; after that, it is placed in the dark at room temperature for 72 hours, or 465nm light radiation (39mW / cm 2 ) After 5 minutes, record the H NMR spectrum of the solution after the light and dark treatment and contrast the H NMR spectrum of the control sample to analyze its dark and photostability properties. Such as figure 2 As shown, the spectrum of the ruthenium complex does not change significantly under the conditions of dark and light treatment, and it can be seen that the ruthenium complex has good dark stability and light stability.
Embodiment 3
[0069] Absorbance and fluorescence intensity measurements of the near-infrared fluorescent ruthenium complex obtained in Example 1 in different solvents
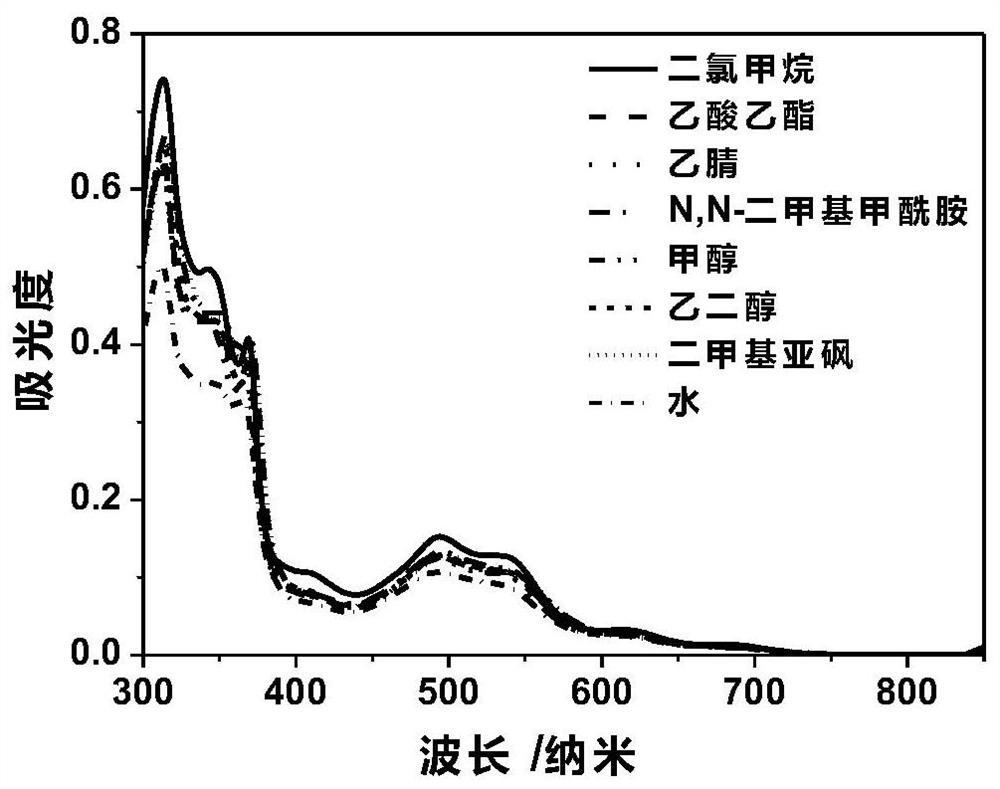
[0070] 1. Absorbance of ruthenium complexes in different solvents
[0071] Using dichloromethane, ethyl acetate, acetonitrile, N,N-dimethylformamide, methanol, ethylene glycol, dimethyl sulfoxide, and water as solvents, the metal ruthenium complex was formulated into a 10 μM sample solution, using A double-beam UV-Vis spectrophotometer records the UV absorption spectrum of the ruthenium complex, such as image 3 Shown represents its absorbance in different solvents. It can be seen from the figure that due to the introduction of the bnp ligand, the compound of the present invention exhibits good optical properties, and the absorption peak of the metal ruthenium complex is red-shifted in the visible light region compared with most existing ruthenium complexes.
[0072] 2. Fluorescence intensity of ruthenium complexes in diff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com