Method for analyzing benserazide impurity A in levodopa and benserazide hydrochloride compound preparation
A compound preparation and dopasehydrazine technology, applied in the field of drug analysis and detection, can solve the problems of low accuracy, low sensitivity at low concentration, unsatisfactory recovery rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Chromatographic conditions:
[0076] Instrument: High performance liquid chromatography equipped with UV detector
[0077] Chromatographic column: Ultimate AQ C18 4.6*250mm 5μm (filler: B-type ultra-high purity fully porous spherical silica gel filler)
[0078] Mobile phase A: Add 2.2g sodium heptanesulfonate + 6.8g potassium dihydrogen phosphate to 1L water, adjust pH to 6.0 with 5M sodium hydroxide
[0079] Mobile Phase B: Methanol
[0080] Detection wavelength: 210nm
[0081] Flow rate: 1.0mL / min
[0082] Injection volume: 10μL
[0083] Column temperature: 30°C
[0084] Sample chamber temperature: 4°C
[0085] Running time: 60min
[0086] time / min Mobile phase A / % Mobile phase B / % 0-15 100 0 15-35 100→30 0→70
[0088] Blank solution preparation:
[0089] with diluent.
[0090] Linear solution 1:
[0091] Weigh an appropriate amount of benserazide impurity A reference substance, dilute with a dil...
Embodiment 2
[0109] Chromatographic conditions: change the pH in the mobile phase A, adjust the pH to 7.0 with 5M sodium hydroxide, and keep other conditions unchanged.
[0111] Resolution solution (system suitability solution):
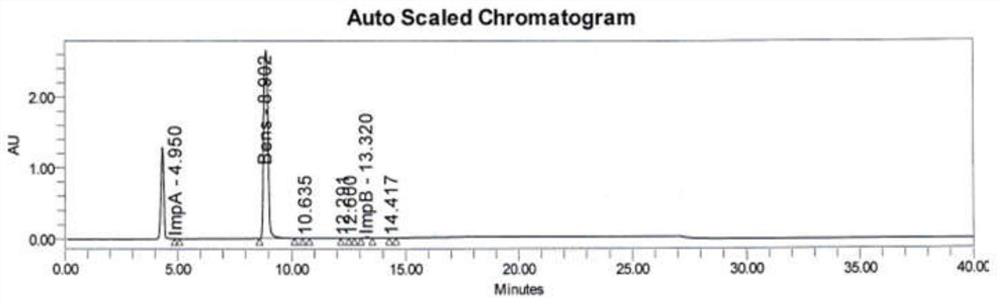
[0112] Get the separation degree solution of embodiment 1 and carry out detection and analysis by above-mentioned detection condition, system suitability chromatogram is shown in Figure 8 . Figure 8 The peak order of each component in the product is levodopa, benserazide impurity A, levodopa impurity B, the peak elution time is 5.988min, 4.686min, 8.195min, and their resolutions are 2.78, 3.88, All chromatographic parameters meet the requirements.
[0113] Accuracy (0.1%) solution:
[0114] Weigh 1000mg of levodopa and 1698mg of blank excipients into a 100ml measuring bottle, accurately measure 1ml of benserazide impurity A accuracy stock solution into the same measuring bottle, add diluent to 2 / 3 volume of the measuring bottl...
Embodiment 3
[0117] Chromatographic conditions: change the pH in the mobile phase A, adjust the pH to 2.0 with phosphoric acid, and keep other conditions unchanged.
[0118] Diluent: 70% Methanol
[0119] Resolution solution (system suitability solution):
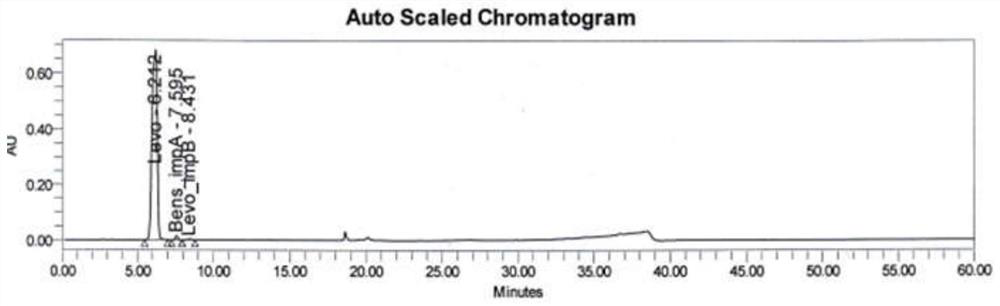
[0120] Get the separation degree solution of embodiment 1 and carry out detection and analysis by above-mentioned detection condition, system suitability chromatogram is shown in Figure 10 . Figure 10 The order of the peaks of each component in the product is levodopa, benserazide impurity A, levodopa impurity B, the peak elution time is 29.710min, 30.141min, 30.722min, and their resolutions are 2.35, 3.38, All chromatographic parameters meet the requirements.
[0121] Accuracy (0.1%) solution:
[0122] Weigh 1000mg of levodopa and 1698mg of blank excipients into a 100ml measuring bottle, accurately measure 1ml of benserazide impurity A accuracy stock solution into the same measuring bottle, add diluent to 2 / 3 volume of the measur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com