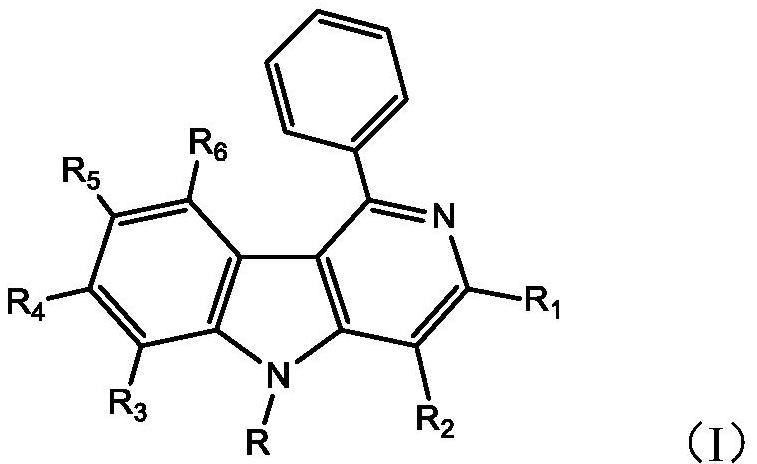
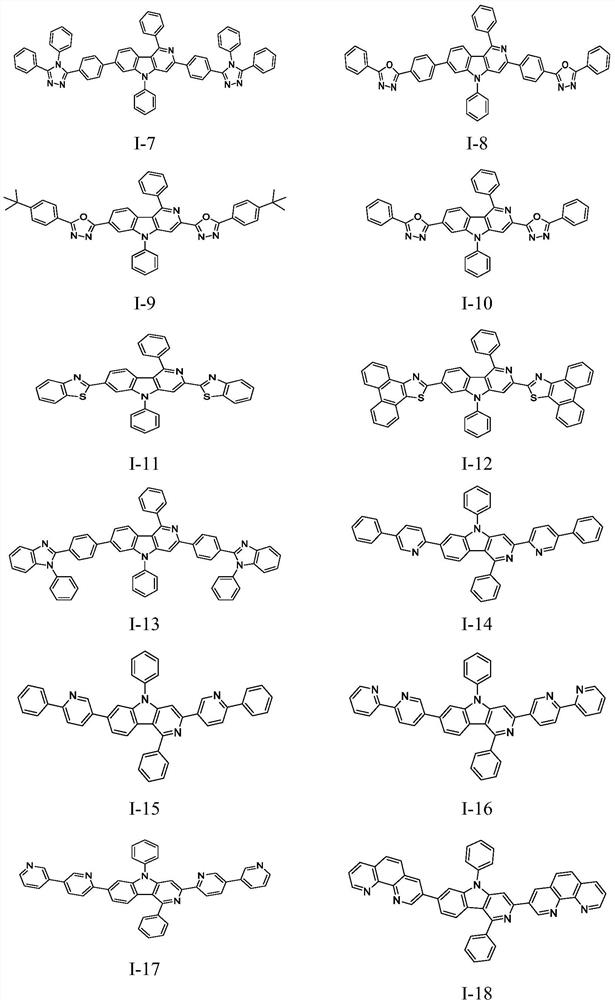
Carbazole ring-containing compound and application thereof
A compound, carbazole ring technology, applied in the field of new carbazole ring-containing compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Synthesis of Intermediate P1
[0056] The synthetic route is:
[0057]
[0058] The synthesis specifically includes the following steps:
[0059] (1) Take a 1 liter three-necked flask with magnetic stirring, add 20.35g (0.189mol) of sodium carbonate, 12g (0.1mol) of phenylboronic acid and 100ml of toluene after nitrogen replacement; add 5g (7mmol) Pd132 after nitrogen replacement; add After finishing, heat to 80℃; then start to add dropwise a solution consisting of 27.72g 5,6-dibromopyridin-2-amine (0.11mol) and 100ml toluene, and control the temperature at 75-90℃; after the reaction, separate The organic phase was extracted, dried, column chromatography, and the solvent was spin-dried to obtain 18.675 g of yellow solid P1-1 with a yield of 75%.
[0060] (2) Take a 1 liter three-necked flask with magnetic stirring. After nitrogen replacement, add 20.35g (0.189mol) of sodium carbonate, 24.5g (0.1mol) of (4-chloro-2-nitrophenyl)boronic acid and 100ml of toluene; After...
Embodiment 2
[0065] Example 2 Synthesis of intermediate P2
[0066] The synthetic route is:
[0067]
[0068] The difference between the preparation method and Example 1 is that in step (3), concentrated hydrobromic acid and cuprous bromide are used instead of concentrated hydrochloric acid and cuprous chloride, respectively, and an appropriate material ratio is selected, and other raw materials and steps are uniform. In the same manner as in Example 1, intermediate P2 was obtained.
[0069] Product MS (m / e): 432; Elemental analysis (C 23 H 14 BrClN 2 ): Theoretical value C: 63.69%, H: 3.25%, N: 6.46%; measured value: C: 63.24%, H: 3.50%, N: 6.15%.
Embodiment 3
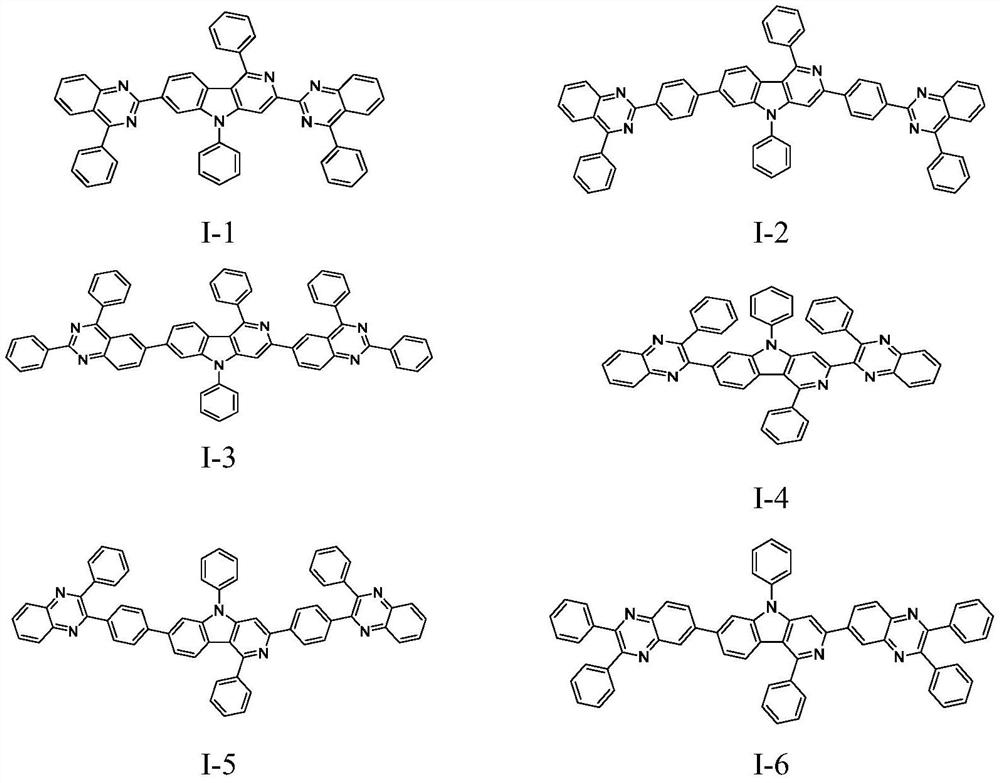
[0070] Example 3 Synthesis of Compound I-1
[0071]
[0072] The synthetic route is as follows:
[0073]
[0074] The synthesis of compound I-1 includes the following specific steps:
[0075] Take a 1000ml three-necked flask with magnetic stirring. After nitrogen replacement, add 38.8g compound P1 (0.1mol), (4-phenylquinazolin-2-yl)boronic acid 52.5g (0.21mol), cesium carbonate (78g, 0.24) mol) and 500ml of dioxane, start stirring. After replacing with nitrogen again, add (0.8g, 4mmol) tri-tert-butylphosphine and (1.4g, 1.5mmol) tris(dibenzylideneacetone)dipalladium. After the addition, heat to 80-90°C and react for 5 hours , TLC monitors that the reaction is complete. Then the temperature was lowered to room temperature, the pH was adjusted to neutral, the organic phase was separated, extracted, dried, column chromatography, and the solvent was spin-dried to obtain 58.96 g of pale yellow solid I-1 with a yield of 81%.
[0076] Product MS (m / e): 728; Elemental analysis (C 51 H 32 N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com