Preparation method of low-impurity and high-solubility basic nickel carbonate
A high solubility, nickel carbonate technology, applied in nickel carbonate and other directions, can solve the problems of poor solubility of molybdenum oxide/phosphoric acid, high solubility, low crystallinity, etc., to improve product uniformity, good stability, and low product impurities. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] A kind of preparation method of the basic nickel carbonate of low impurity, high solubility of the present invention, comprises the following steps:
[0019] (1) preparation contains the salt solution of nickel ion; The nickel salt in the salt solution containing nickel ion is one or more in nickel chloride, nickel sulfate, nickel nitrate; Concentration of nickel ion in the salt solution containing nickel ion It is 120g / L-130g / L.
[0020] (2) prepare salt solution containing carbonate; the concentration of carbonate in the salt solution containing carbonate is 200g / L-220g / L. Preferably, the salt solution containing carbonate is sodium carbonate solution.
[0021] (3) The salt solution containing nickel ions in step (1) and the salt solution containing carbonate in step (2) are respectively passed through a large centrifugal pump to adopt a parallel flow mode large flow rate, and quickly add to the reaction kettle for coprecipitation Reaction, reaction temperature, sti...
Embodiment 1
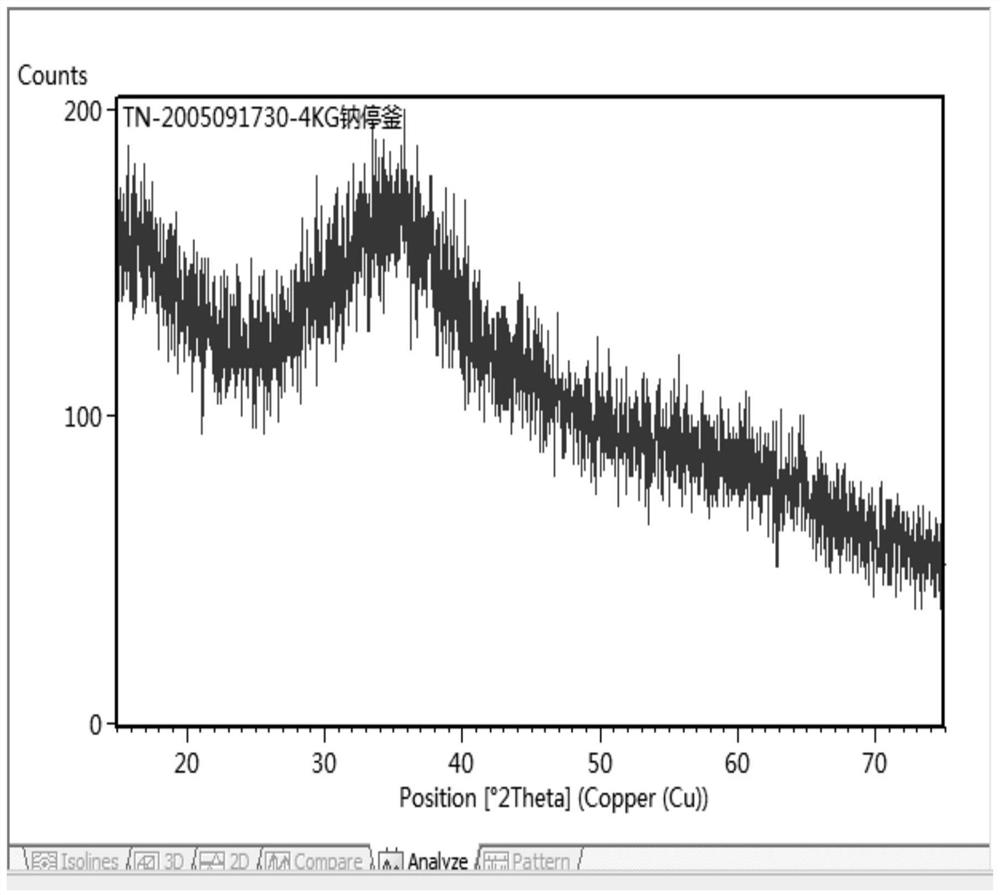
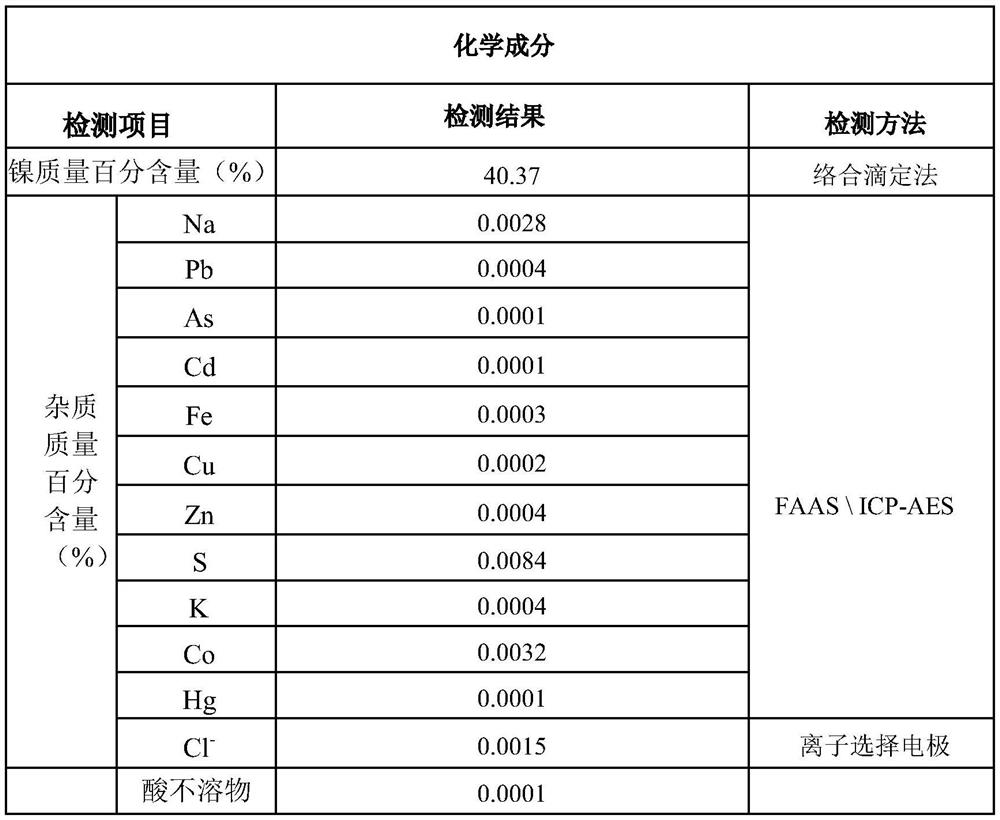
[0026] Add nickel ion-containing salt solution with a nickel ion concentration of 120g / L and carbonate-containing salt solution with a carbonate concentration of 220g / L to the 6m 3 Co-precipitation reaction was carried out in the reactor to obtain the mixture, and the diameter of the stirring blade of the reactor was 660mm; the reactor was double-layer stirred, and the feed pipe of the reactor was located at the top of the reactor. The feed flow rate of the salt solution containing nickel ions added to the reactor is 300kg / h, the feed rate of the salt solution containing carbonate radicals added to the reactor is 420kg / h, and the process conditions of the co-precipitation reaction are: The pH is 8.2-8.4, the reaction temperature is 50° C., the reaction time is 7 minutes, and the reaction speed is 170 r / min. Put the mixture into a filter press for two filter press washes to obtain a basic nickel carbonate wet material; the water temperature for the filter press wash is 50°C, an...
Embodiment 2
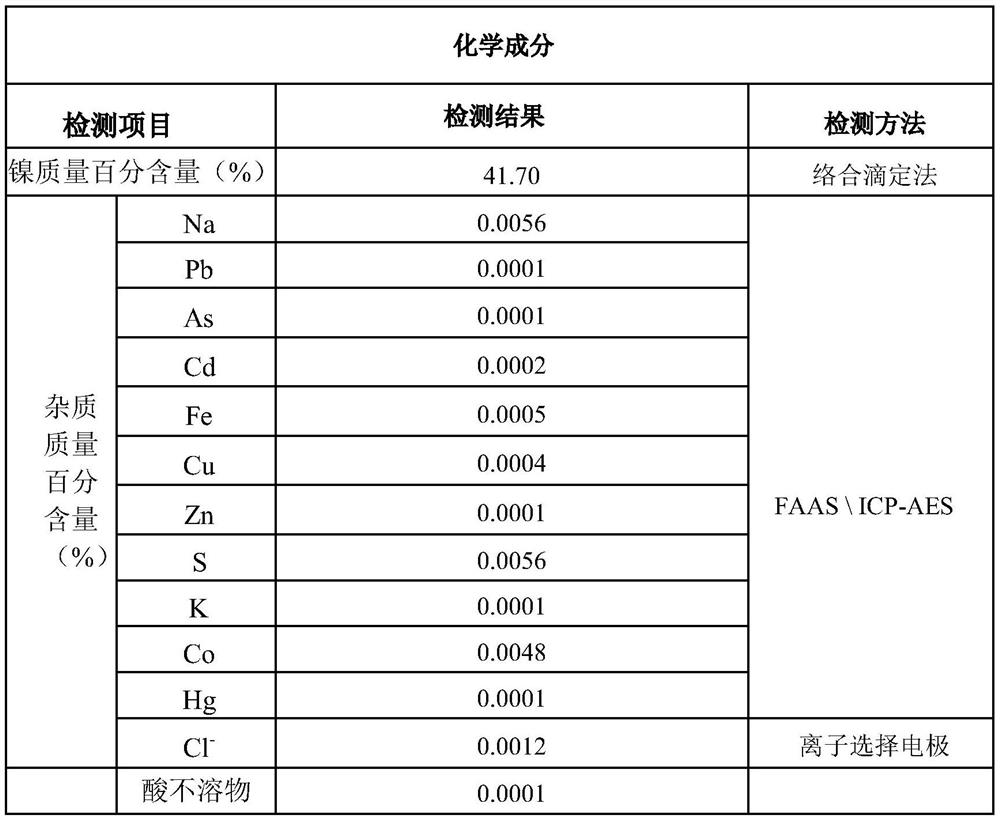
[0030] Add nickel ion-containing salt solution with a nickel ion concentration of 130g / L and carbonate-containing salt solution with a carbonate concentration of 200g / L to the 6m 3 Co-precipitation reaction is carried out in the reactor to obtain the mixture, the diameter of the stirring blade of the reactor is 680 mm; the reactor is double-layer stirring, and the feed pipe of the reactor is located at the top of the reactor. The feed flow rate of the salt solution containing nickel ions added to the reactor is 300kg / h, the feed rate of the salt solution containing carbonate radicals added to the reactor is 480kg / h, and the process conditions of the co-precipitation reaction are: The pH is 8.2-8.35, the reaction temperature is 53° C., the reaction time is 8 minutes, and the reaction speed is 190 r / min. Put the mixture into a filter press for two filter press washes to obtain a basic nickel carbonate wet material; the water temperature for the filter press wash is 55°C, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com