Core-shell-shaped copper selenide and ferronickel hydrotalcite electrocatalyst and preparation method and water electrolysis application thereof
A technology of copper selenide and catalyst, applied in the field of electrocatalysis, to achieve the effect of uniform size, good mechanical stability and thin sheet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
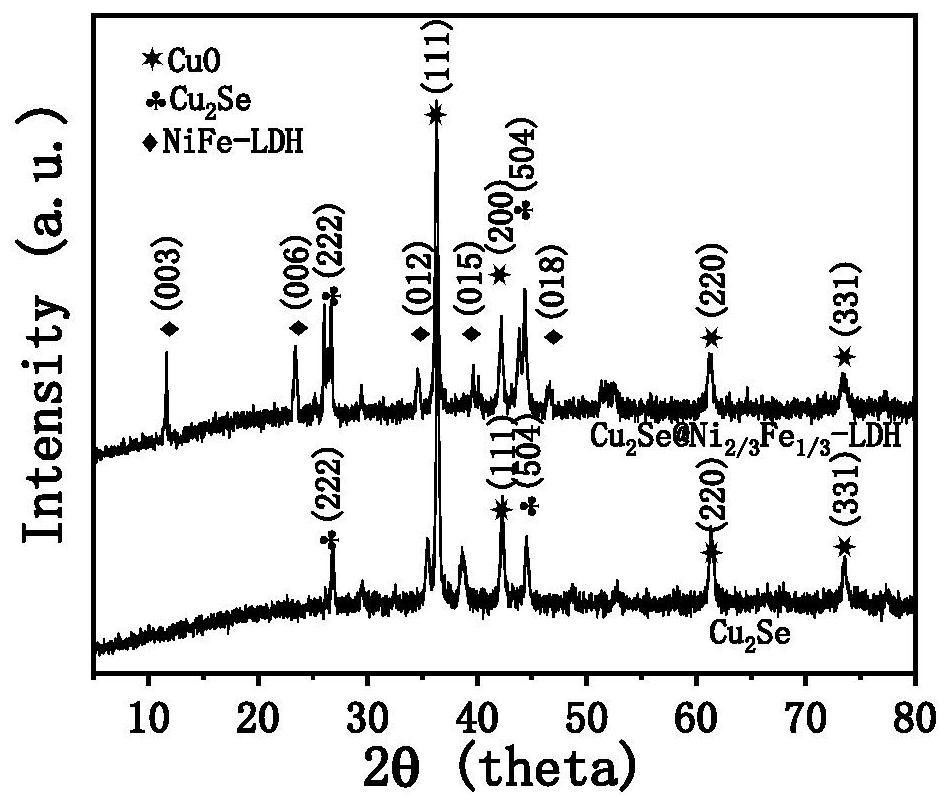
[0034] (1)Cu 2 Preparation of Se / CF
[0035] Soak the commercial copper foam with a specification of 3cm×4cm in a hydrochloric acid solution with a concentration of 37% for 10 minutes, and wash it several times with deionized water and absolute ethanol; put the cleaned foam copper into 80mL NaOH and (NH 4 ) 2 S 2 o 8 Soaked in the mixed solution for 20min to grow light blue Cu(OH) on the surface in situ 2 nanowires, grown with Cu(OH) 2 The copper foam of the nanowires was rinsed with deionized water, and dried in an oven at 60 °C for 6 h; the dried Cu(OH) 2 The nanowires are placed in a tube furnace, and 0.1g of selenium powder is placed at the front of the tube furnace, and the temperature is raised to 400 °C at a rate of 5 °C / min in a nitrogen atmosphere, and kept for 30 min. After the tube furnace cools down naturally, the sample is taken out , washed several times with deionized water and ethanol to obtain Cu 2 Se / CF, dry for later use;
[0036] (2)Cu 2 Preparatio...
Embodiment 2
[0039] (1)Cu 2 Preparation of Se / CF
[0040] Prepare with reference to the method and conditions of step (1) in Example 1.
[0041] (2)Cu 2 Preparation of Se@NiFe-LDH / CF
[0042] Referring to the method and preparation conditions of step (2) in Example 1, the electrodeposition time was set to 60s to obtain copper selenide@nickel-iron hydrotalcite nanosheet electrocatalyst, denoted as Cu 2 Se@Ni 2 / 3 Fe 1 / 3 - LDH / CF-60;
Embodiment 3
[0044] (1)Cu 2 Preparation of Se / CF
[0045] Prepare with reference to the method and conditions of step (1) in Example 1.
[0046] (2)Cu 2 Preparation of Se@NiFe-LDH / CF
[0047] Referring to the method and preparation conditions of step (2) in Example 1, the electrodeposition time was set to 90s to obtain copper selenide@nickel-iron hydrotalcite nanosheet electrocatalyst, denoted as Cu 2 Se@Ni 2 / 3 Fe 1 / 3 - LDH / CF-90;
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com