Slow-release modification method of solid electrolyte/metal lithium interface and solid-state lithium metal battery
A technology of solid electrolyte and metal lithium, which is applied in the direction of solid electrolyte, electrolyte storage battery manufacturing, non-aqueous electrolyte storage battery, etc., can solve the problems of no practical value, low lithium ion conductivity, affecting charge and discharge performance, etc., to improve capacity stability And the effects of cycle stability, high Li-ion transport capacity, and ease of large-scale application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
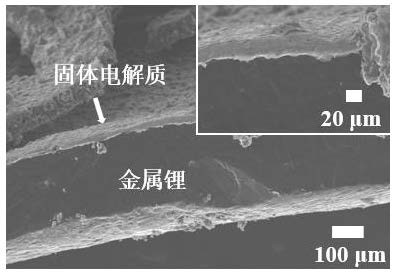
Image
Examples
Embodiment 1
[0066] Step (1): Polypropylene carbonate and lithium bistrifluoromethanesulfonimide were added into acetonitrile solvent at a mass ratio of 6:1 and stirred until completely dissolved, and prepared to obtain a solution with a concentration of 30 wt%. 40 μL of the above solution was uniformly coated on a metal lithium disc (14 mm in diameter) and dried in vacuum to obtain a lithium metal negative electrode with a self-degradable polymer protective layer on the surface.
[0067] Step (2): Activate the lithium metal negative electrode at 60°C until the polymer layer on its surface presents a viscous gel-like shape, and attach it to the cold-pressed silver-argentite-type sulfide Li 6 P.S. 5 Cl solid electrolyte sheet (disk with a diameter of 14mm) on one side, and then cooled naturally.
[0068] Step (3): In the argentite-type sulfide Li 6 P.S. 5 The other side of the Cl solid electrolyte sheet also carries out the operations of step (1) and step (2). Finally, steel sheets (dis...
Embodiment 2
[0071] It is basically the same as Example 1, the only difference is:
[0072] Step (1): Polypropylene carbonate and lithium bistrifluoromethanesulfonimide were added into acetonitrile solvent at a mass ratio of 6:1 and stirred until completely dissolved, and a solution with a concentration of 40 wt% was prepared. 40 μL of the above solution was uniformly coated on a metal lithium disc (14 mm in diameter) and dried in vacuum to obtain a lithium metal negative electrode with a self-degradable polymer protective layer on the surface.
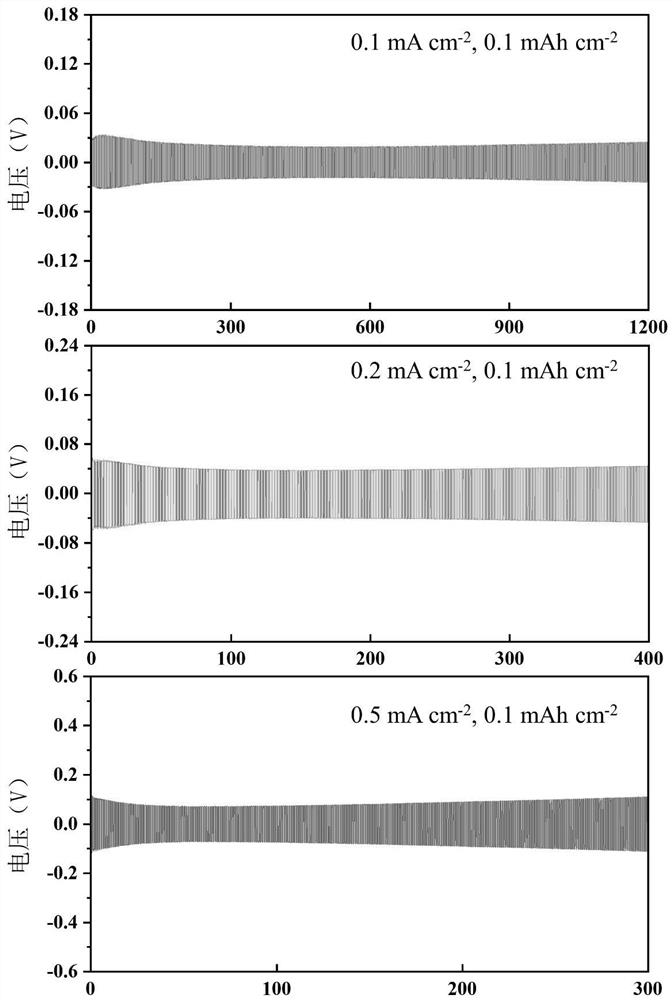
[0073] Battery performance evaluation: Conduct potentiostatic electrochemical AC impedance spectroscopy tests on lithium symmetrical batteries at 25°C, with frequencies ranging from 0.1Hz to 1MHz, such as image 3 As shown, the interface impedance is about 97ohm / cm 2 . The slow-release polarization of the symmetric battery decreases in the early stage during the cycle, and then the polarization is stable, and its interface stability is generally...
Embodiment 3
[0075] It is basically the same as Example 1, the only difference is:
[0076] Step (1): Polypropylene carbonate and lithium bistrifluoromethanesulfonimide were added into the acetonitrile solvent at a mass ratio of 6:1 and stirred until completely dissolved, and a solution with a concentration of 50 wt% was prepared. 40 μL of the above solution was uniformly coated on a metal lithium disc (14 mm in diameter) and dried in vacuum to obtain a lithium metal negative electrode with a self-degradable polymer protective layer on the surface.
[0077]Battery performance evaluation: Conduct potentiostatic electrochemical AC impedance spectroscopy tests on lithium symmetric batteries at 25°C, with frequencies ranging from 0.1Hz to 1MHz, and the interface impedance is about 95ohm / cm 2 . The slow-release polarization of the symmetric battery decreases in the early stage during the cycle, and then the polarization is stable, and its interface stability is generally better.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com