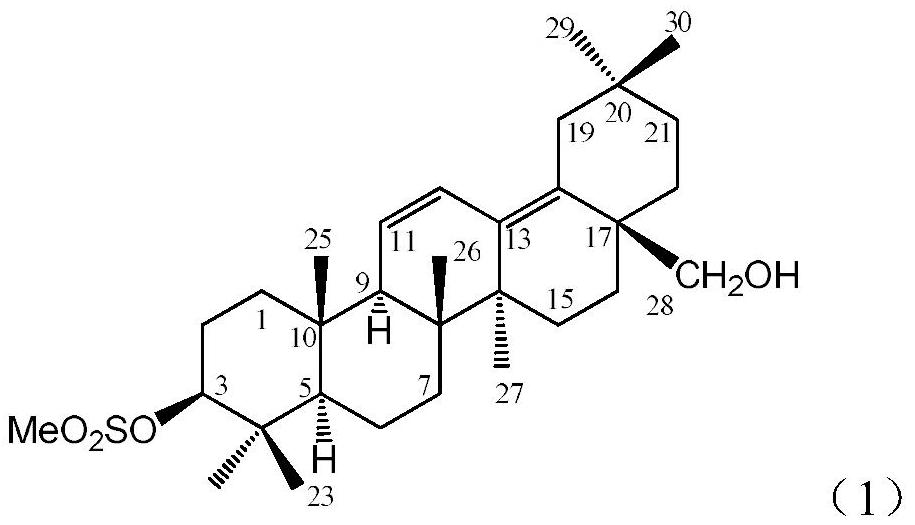
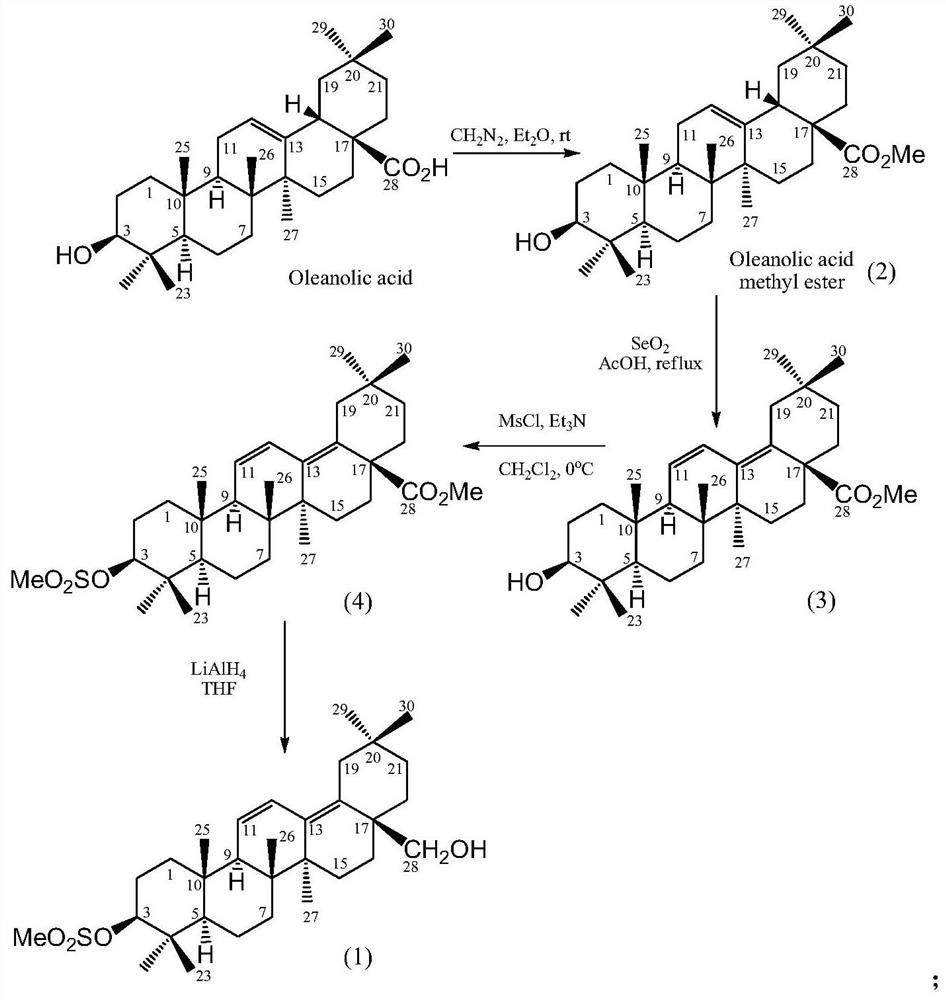
Preparation of dienyl mesyloxy oleanane alcohol and medical application of dienyl mesyloxy oleanane alcohol in resisting hepatitis B
A technology of diene methanesulfonyloxy oleanane and methanesulfonyloxy oleanane, which is applied in the field of preparation of diene methanesulfonyloxy oleanol, can solve the problems that have not been effectively developed and other issues, to achieve the effects of clear industrialization prospects, inhibition of duplication, and wide distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 : the preparation of formula (1) compound 3-methylsulfonyloxy oleanane 11,13 (18)-diene-28-alcohol 1.1 instruments and reagents
[0031] The ultraviolet spectrum was measured with a Shimadzu UV-240 ultraviolet spectrophotometer; the nuclear magnetic resonance spectrum was measured by an INOVA type superconducting nuclear magnetic resonance spectrometer (VARIAN INOVA-400MHz) (tetramethylsilyl ether TMS was the internal standard); electrospray mass spectrometry ESI- MS was determined by Bruker Esquire 3000+ mass spectrometer; silica gel for column chromatography (100-200, 200-300 and 300-400 mesh) and silica gel GF254 for thin-layer chromatography (10-40 mesh) were all produced by Qingdao Ocean Chemical Factory The reagents used are all analytically pure, wherein petroleum ether boiling range is 60~90 ℃; High performance liquid phase detection (HPLC) uses Agilent 1100 instrument; Preparative thin layer chromatography (PTLC) uses the aluminum foil silica gel plat...
Embodiment 2
[0042] Example 2 : the inhibitory effect of formula (1) compound on hepatitis B surface antigen (HBsAg)
[0043] 2.1 Cell culture:
[0044] HepG2.2.15 cells were cultured in DMEM medium containing 10% inactivated fetal bovine serum, 100 U / ml penicillin and 100 U / ml streptomycin, and 100 μg / ml G418 at 37°C, 5% CO 2 , cultured in an incubator with 100% relative humidity.
[0045] 2.2 Determination of the inhibitory effect of the test sample on the HBsAg secreted by HepG2.2.15 cells:
[0046] Take the HepG2.2.15 cells in the logarithmic growth phase, and dilute the cells to 1×10 with medium 5 / ml, seeded in 96-well cell culture plate, 100ml per well, at 37°C, 5% CO 2 After culturing in an incubator with 100% relative humidity for 24 hours, add the test sample diluted with the medium, set up three replicate holes for each concentration, 200 microliters per hole, place at 37°C, 5% CO 2 , cultivated in an incubator with 100% relative humidity, change the culture medium contain...
Embodiment 3
[0053] Example 3 : the inhibitory effect of formula (1) compound on hepatitis B e antigen (HBeAg)
[0054] 3.1 Cell culture: the method is the same as in Example 2.
[0055] 3.2 Determination of the inhibitory effect of the test sample on the HBeAg secreted by HepG2.2.15 cells:
[0056] Take the HepG2.2.15 cells in the logarithmic growth phase, and dilute the cells to 1×10 with medium 5 / ml, seeded in 96-well cell culture plate, 100ml per well, at 37°C, 5% CO 2 After culturing in an incubator with 100% relative humidity for 24 hours, add the test sample diluted with the medium, set up three replicate holes for each concentration, 200 microliters per hole, place at 37°C, 5% CO 2 , cultivated in an incubator with 100% relative humidity, change the culture medium containing the same concentration sample every 4 days, mix the same volume of the culture medium with the same concentration of the same sample and the same concentration, and use it as the sample to be tested. On t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com