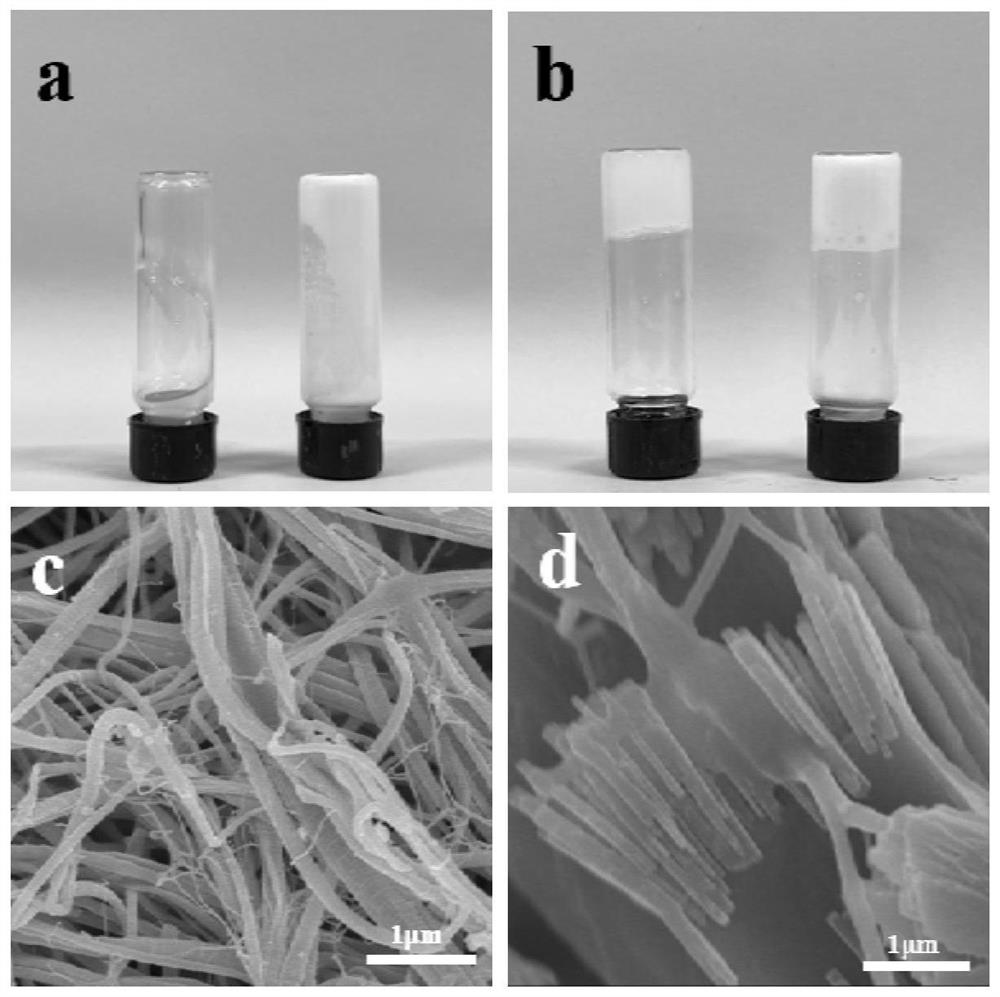
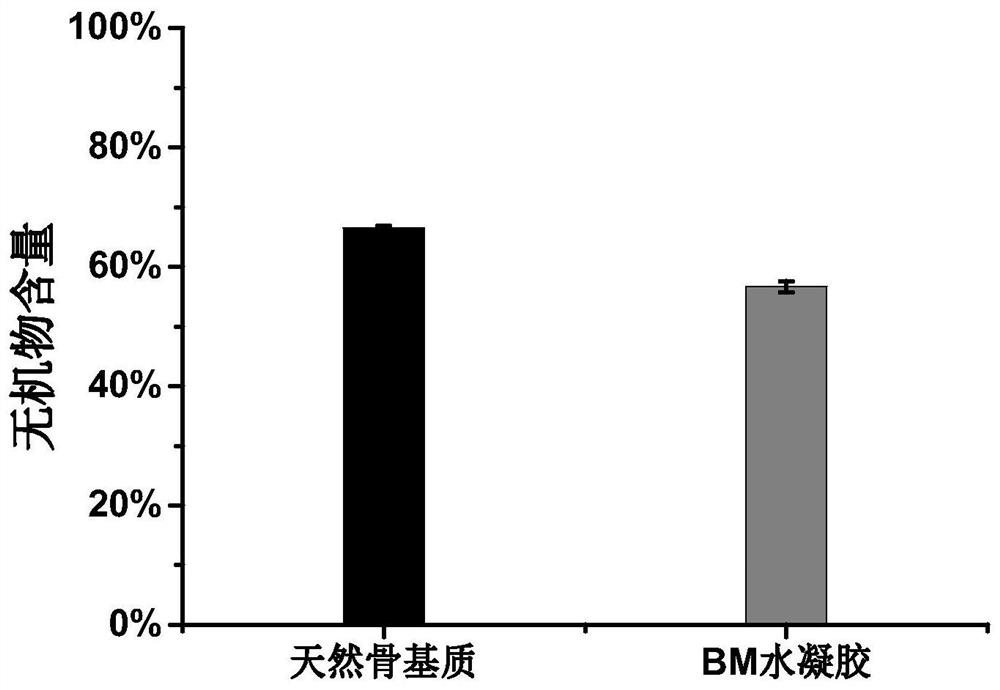
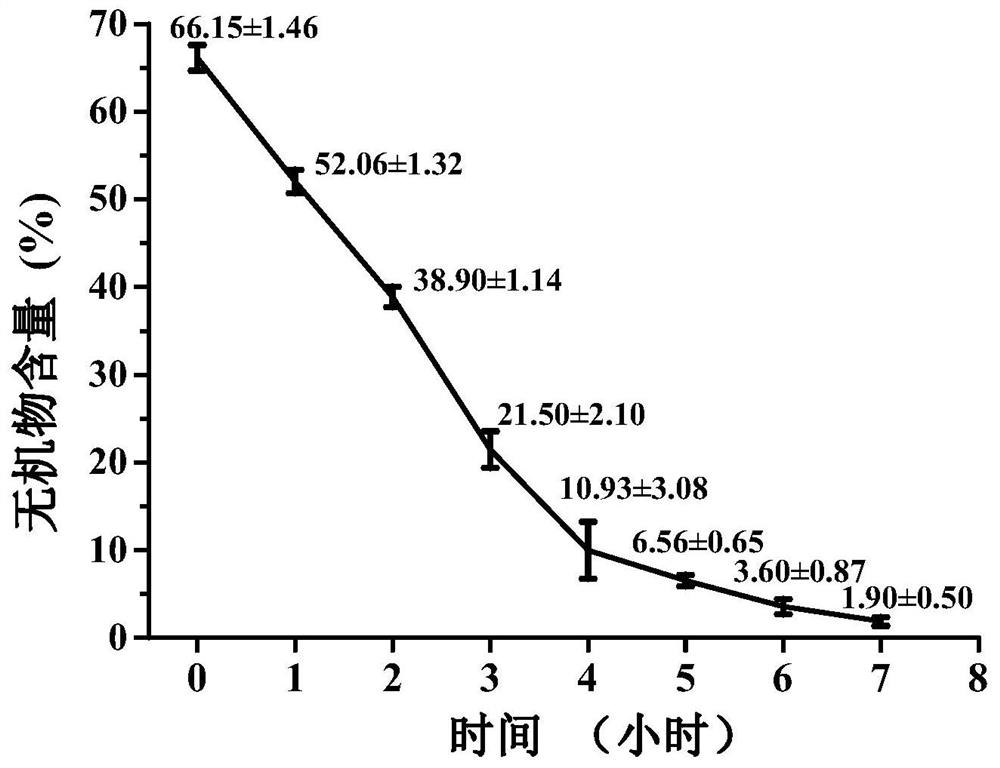
Acellular bone matrix hydrogel with reserved natural hydroxyapatite and preparation method of acellular bone matrix hydrogel
A kind of acellular bone matrix, hydroxyapatite technology, applied in medical science, prosthesis, tissue regeneration and other directions, can solve the problems of easy agglomeration, uneven dispersion, limited addition of hydroxyapatite, etc., to achieve good storage stability The effect of stability, uniform dispersion and good injectability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] A method for preparing a decellularized bone matrix hydrogel retaining natural hydroxyapatite, comprising the following steps:
[0061] a. Choose fresh sheep cortex bone, rinse repeatedly with high-speed water to remove bone marrow, freeze-dry for 24 hours, crush, and sieve to obtain bone powder with a particle size of less than 0.1 mm. Soak the bone powder in 75% ethanol for 1 hour to sterilize, and wash with PBS buffer. 3 hours;
[0062] b. Soak the bone powder obtained in step a with a mixture of chloroform and methanol at a volume ratio of 1:1 for 2 h, the ratio of bone powder to chloroform / methanol mixture solution is 1:25 (w / v), and wash with PBS buffer for 3 hours Then soak the bone powder in pH 7.4 10 mM Tris-HCl buffer solution for 12 hours, the mass volume ratio of bone powder and Tris-HCl buffer solution is 1:25 (w / v) to obtain defatted bone powder;
[0063] c. Soak the defatted bone powder prepared in step b in 10 mM Tris-HCl buffer solution containing 0.8%...
Embodiment 2
[0069] A method for preparing a decellularized bone matrix hydrogel retaining natural hydroxyapatite, comprising the following steps:
[0070] a. Choose fresh bovine cortical bone, rinse repeatedly with high-speed water to remove bone marrow, freeze-dry for 24 h, crush, and sieve to obtain bone powder with a particle size of less than 1 mm. Soak the bone powder in 75% ethanol for 0.5 h to sterilize, and buffer with PBS. Wash with liquid for 6 h;
[0071] b. Soak the bone meal obtained in step a for 3 hours in a mixture of chloroform and methanol with a volume ratio of 1:0.5, the ratio of bone meal to chloroform / methanol mixture solution is 1:25 (w / v), and wash with PBS buffer for 3 hours Then soak the bone powder in pH 7.4 10 mM Tris-HCl buffer solution for 14 hours, the mass volume ratio of bone powder and Tris-HCl buffer solution is 1:25 (w / v) to obtain defatted bone powder;
[0072] c. Soak the defatted bone powder prepared in step b in pH 7.4 10 mM Tris-HCl buffer contain...
Embodiment 3
[0079] A method for preparing a decellularized bone matrix hydrogel retaining natural hydroxyapatite, comprising the following steps:
[0080] a. Select fresh sheep cancellous bone, rinse repeatedly with high-speed water to remove bone marrow, freeze-dry for 24 hours, pulverize, and sieve to obtain bone powder with a particle size of less than 0.1 mm. Soak the bone powder in 75% ethanol for 1.5 h to sterilize, and wash with PBS Wash with buffer for 1 h;
[0081] b. Soak the bone meal obtained in step a in the mixed solution of chloroform and methanol with a volume ratio of 1:2 for 1 hour, the ratio of bone meal and chloroform / methanol mixed solution is 1:25 (w / v), and wash with PBS buffer for 3 times ; Soak the bone powder in pH 7.4 10 mM Tris-HCl buffer solution for 10 h, the mass volume ratio of bone powder and Tris-HCl buffer solution is 1:25 (w / v), to obtain defatted bone powder;
[0082] c. Soak the defatted bone powder prepared in step b in pH 7.4 10 mM Tris-HCl buffer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com