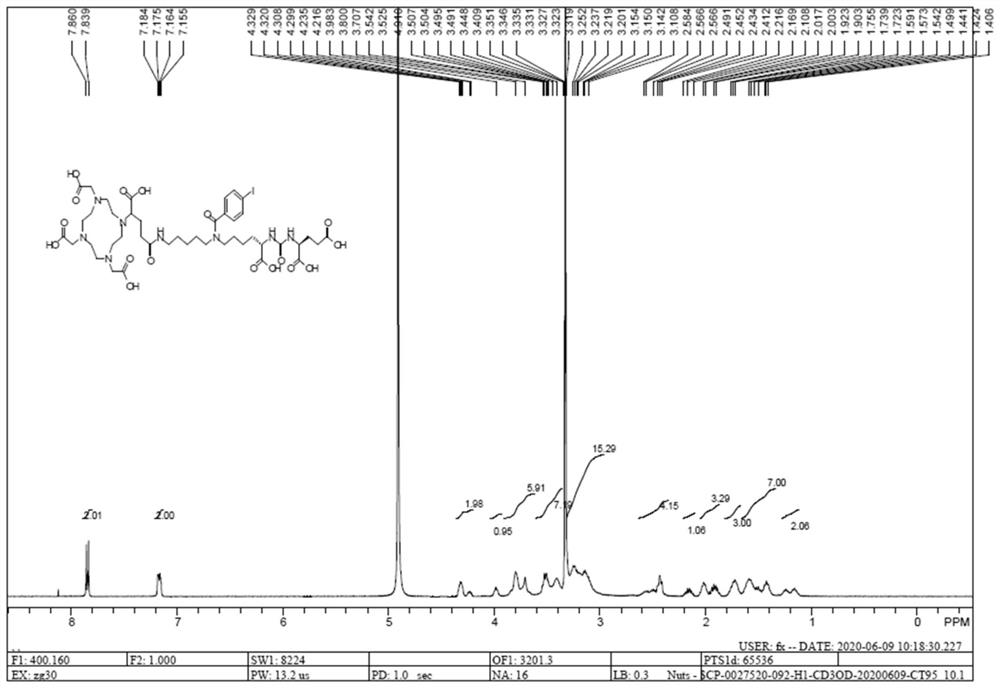
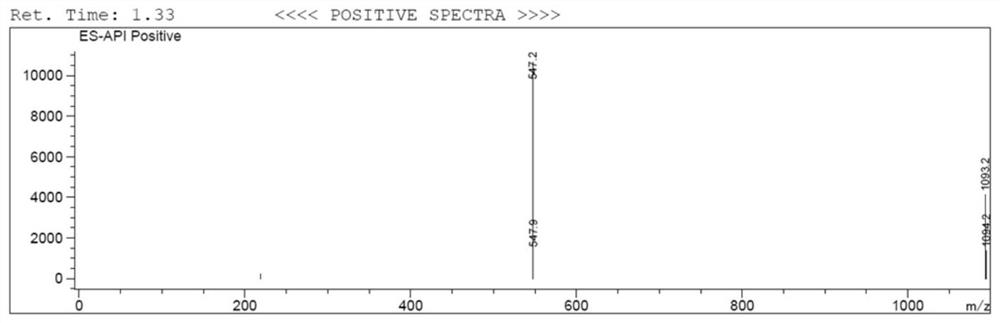
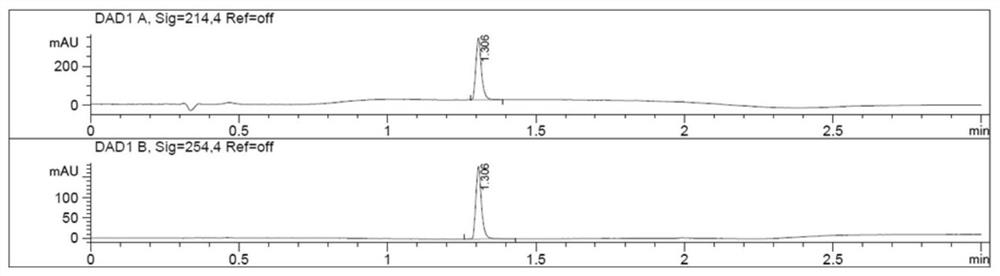
Prostate-specific membrane antigen inhibitor, its metal marker, its preparation method and application
A prostate-specific, membrane antigen technology, applied in the field of biomedicine, can solve the problems of insufficient cell internalization rate and cell uptake rate, change in inhibitor affinity, and the structure-activity relationship is yet to be established, etc. Easy, mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] This embodiment discloses the synthesis of compound S-1, and its reaction formula is:
[0098]
[0099] Specifically: add triethylamine (4.11g, 40.7mmol) to a solution of S (6.0g, 20.3mmol) in dichloromethane (50mL) at room temperature, then add triphosgene (2.00g, 6.71mmol), and react for 30 Minutes later, additional R1 (5.30 g, 14.2 mmol) and triethylamine (1.44 g, 14.2 mmol) were added. The mixture was stirred at room temperature for 16 hours. The reaction was followed by TLC. The reaction mixture was poured into 200 mL of ice water, extracted with ethyl acetate (3×60 mL), washed with brine three times, and dried over anhydrous sodium sulfate for two hours. The organic solvent was removed by rotary evaporation under reduced pressure, and then purified by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain 7.5 g of yellow oily compound, yield: 59.4%.
Embodiment 2
[0101] This embodiment discloses the synthesis of compound S-2, and its reaction formula is:
[0102]
[0103] Get the ethyl acetate (100mL) mixture of compound S-1 (4.0g, 6.44mmol) and Pd / C (0.322mmol) prepared by the method of Example 1 in 1 atm atmospheric pressure of H 2 Stir at room temperature for 16 hours. Filter out unreacted Pd / C. The filtrate was collected, ethyl acetate was distilled off under reduced pressure, and the obtained oily liquid was passed through a silica gel column (mobile phase: petroleum ether / ethyl acetate=1 / 1) 2.7 g of dark green oil, yield: 86.1%.
Embodiment 3
[0105] This example discloses the synthesis of compound S-3-3 (i.e. in compound S-3, X=3), and its reaction formula is:
[0106]
[0107]. Take compound S-2 (2.5g, 5.13mmol) prepared by the method of Example 2, R2 (2.27g, 7.70mmol) and potassium carbonate (1.42g, 10.3mmol) were mixed and dissolved in N,N-dimethyl Formamide (40 mL), stirred at 60°C for 16 hours. After the reaction was completed, the reaction mixture was poured into ice water, extracted with ethyl acetate and washed 3 times with saturated brine. The organic phase was dried over anhydrous sodium sulfate for two hours. After removing the desiccant by filtration, the organic phase was concentrated under reduced pressure, and then purified by a silica gel column (petroleum ether / ethyl acetate=1:1) to obtain 2.8 g of a yellow solid product with a yield of 77.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com