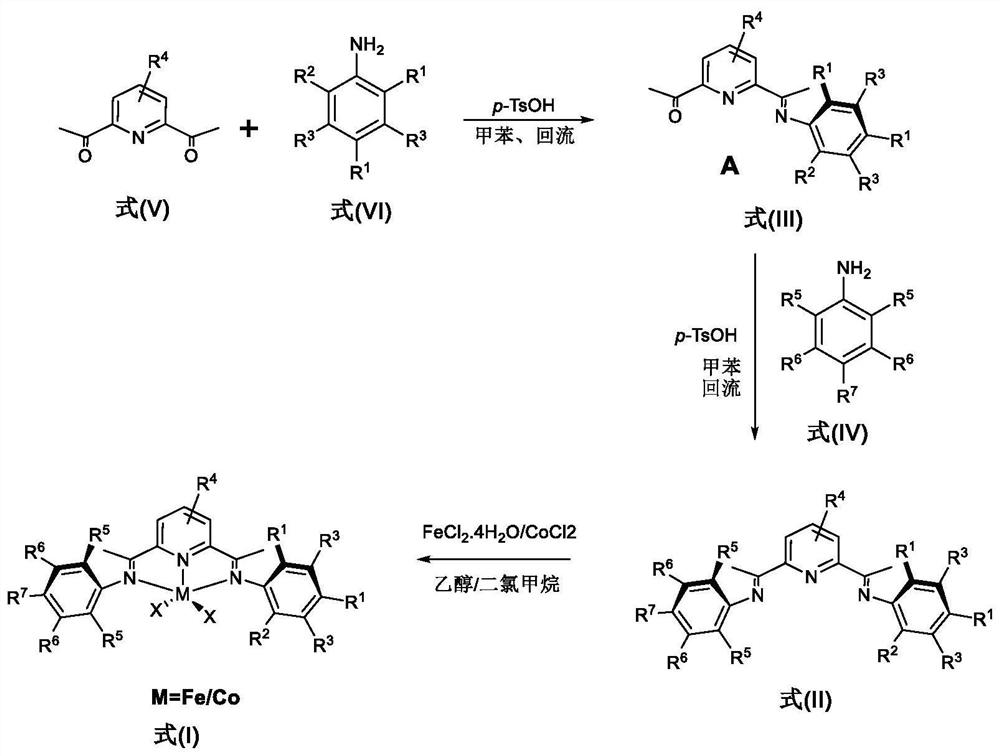
Asymmetric pyridine imine coordination compounds containing large steric hindrance substituent groups, preparation method and application thereof
A technology of complexes and metal complexes, which is applied to compounds containing Group 8/9/10/18 elements of the periodic table, iron-group organic compounds without C-metal bonds, and iron-organic compounds, which can solve post-transition problems Problems such as poor thermal stability of metal complexes, limited molecular weight range of polyethylene materials, and reduced catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
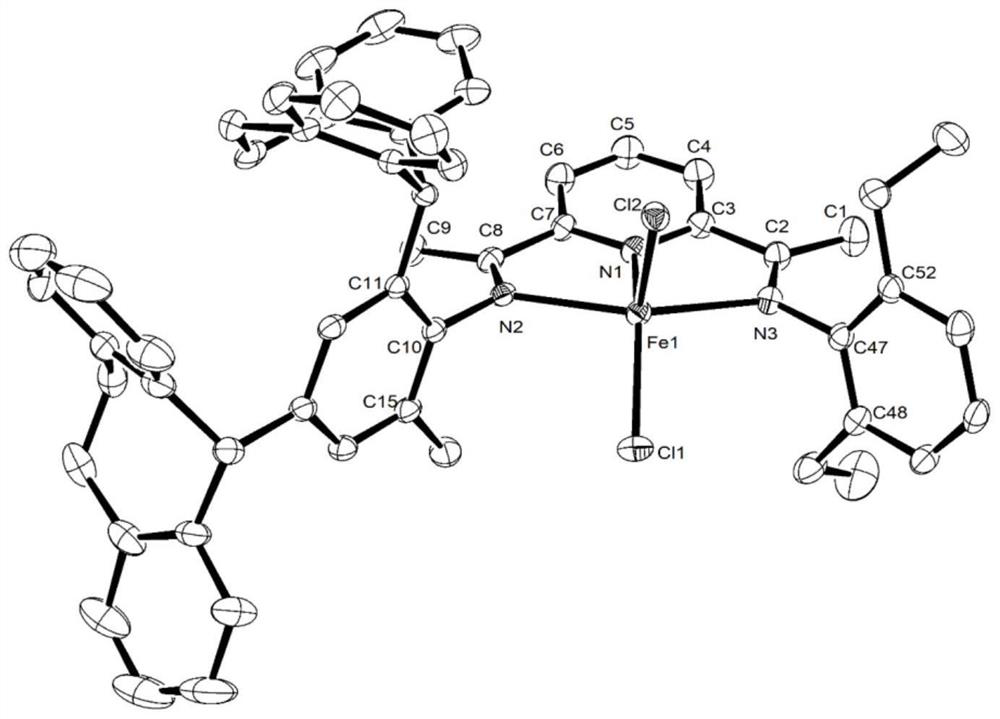
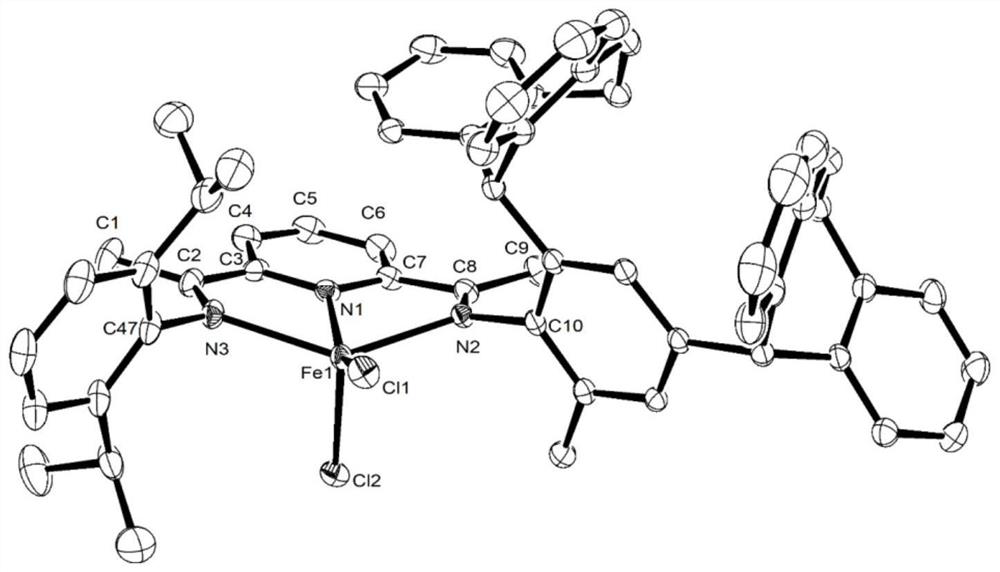
Image
Examples
Embodiment 1
[0129] Preparation of 2-acetyl-6(1-(2,4-bis(dibenzocycloheptyl)-6-methyl-anilino)ethyl)pyridine (A) shown in the following formula
[0130]Weigh 2.13g (13.10mmol) of 2,6-diacetylpyridine and 6.42g (13.10mmol) of 2,4-bis(dibenzocycloheptyl)-6-methyl-aniline into a 15% In 100 mL of toluene solution of p-toluenesulfonic acid, after stirring at reflux temperature for 10 h, the reaction mixture was filtered under heating conditions, and all volatiles were evaporated under reduced pressure conditions. Next, the unprocessed product obtained was dissolved in dichloromethane, loaded with basic alumina, and subjected to column chromatography through a silica-based alumina column, using a mixed solvent of petroleum ether and ethyl acetate as an eluent (25 / 2) carries out elution, detects elution fraction by thin-layer chromatography, developing agent is the mixed solvent that the volume ratio of sherwood oil and ethyl acetate is 10:1, collects the 3rd fraction, removes solvent, obtains 3....
Embodiment 2
[0136] Example 2. Preparation of 2-(1-(2,4-bis(dibenzocycloheptyl)-6-methyl-anilino) ethyl)-6(1-(2, 6-Dimethyl-anilino)ethyl)pyridine (ligand L1)
[0137] Weigh 1.10g (1.70mmol) 2-acetyl-6(1-(2,4-bis(dibenzocycloheptyl)-6-methyl-anilino)ethyl)pyridine (compound A), Add it into the reaction flask, then add catalytic equivalent of p-toluenesulfonic acid (15%) into the reaction flask, and add it into 100mL of toluene solvent to form a solution containing A. 0.21 g (1.70 mmol) of 2,6-dimethylaniline was dropped into the reactor solution containing A. The reaction mixture was heated to reflux for 8h. After the completion of the reaction was detected by TLC, it was cooled to room temperature, and the volatiles were evaporated under reduced pressure. Then, the obtained unprocessed residual solid was dissolved in dichloromethane, loaded with basic alumina, and carried out column chromatography through a silica basic alumina column (using a mixed solvent of petroleum ether and ethyl...
Embodiment 3
[0144] Example 3. Preparation of 2-(1-(2,4-bis(dibenzocycloheptyl)-6-methyl-anilino) ethyl)-6(1-(2, 6-Diethyl-anilino)ethyl)pyridine (ligand L2)
[0145] Basically the same as the method in Example 2, the difference is: in the case of maintaining the same molar number of reactants, the aniline compound reacted with solution A is 2,6-diethylaniline, and after drying, 0.34g of yellow powder is obtained, which is Ligand L2, 2-(1-(2,4-bis(dibenzocycloheptyl)-6-methyl-anilino)ethyl)-6(1-(2,6-diethyl- Anilino)ethyl)pyridine, yield: 26%. Melting point: 215–217°C.
[0146]
[0147] The structural confirmation data are as follows:
[0148] FTIR (KBr, cm-1): 3059(w), 3015(w), 2935(w), 2876(w), 2824(w), 1643(υ(C=N),s), 1569(υ( C=N),w),1489(w),1448(m),1363(m),1302(w),1242(w),1219(w),1197(w),1119(m),1100( w), 1076(w), 1050(w), 1014(w), 963(w), 910(w), 871(w), 849(w), 802(w), 787(w), 759( s), 704(w).
[0149] 1H NMR (400MHz, CDCl 3 .TMS): δ8.45(d, J=8.02Hz, 2H, Py–H), 7.94(t, J=7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| aggregation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com