Fully humanized trivalent specific antibody for neutralizing tetanus toxin and preparation method thereof
A tetanus toxoid, fully humanized technology, applied in the field of genetic engineering, can solve the problems of no clone expression of the sequence, no production application value, and little antibody expression, etc., so as to reduce production costs and reduce human allergy. , the effect of significant economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
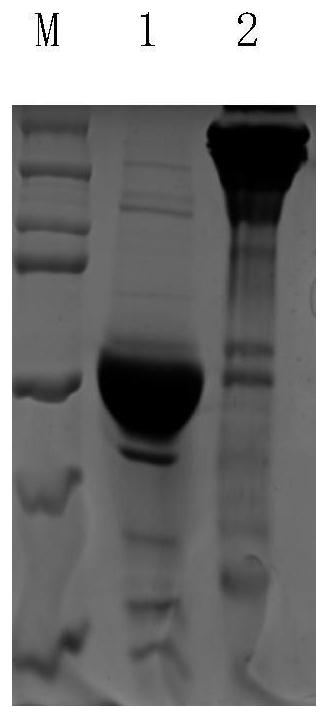
[0017] Example 1 Recombinant expression and purification of glycosidase.
[0018] 1.1 Construction of glycosidase expression sequence plasmid
[0019] The nucleotide sequence (SEQ ID NO: 1) of a-1,2mannosidase (Genebank: XP_006962575) of Trichoderma reesei, encoding 526 amino acids, was biosynthesized by Shanghai Jierui, and then MscI was added to the 5 end of the sequence Restriction site and 3 ends add XhoI restriction site, these sequences are loaded into pET20a vector at last and are transformed in Escherichia coli BL21 (DE) 3, the product obtained after expression is named 6*His-TrMannl (sequence 1 , SEQ ID NO: 1), because it contains Trx and 6*His tags, its molecular weight is about 67KD.
[0020] SEQ ID NO: 1 Trichoderma reesei a-1,2mannosidase (Genebank: XP_006962575) nucleotide sequence (Genebank: XM_006962513), CATATG boxed at the 5-end is NdeI restriction site, CTCGAG boxed at the 3-end is XhoI restriction site location.
[0021] AGATTCCCTAGCAGCTCCGTCCTTGCCCTCGG...
Embodiment 2
[0026] Example 2 Obtaining of Fully Humanized Sequence
[0027] 2.1 Acquisition of ScFv sequence
[0028] According to the previous research of the applicant, after optimization, the following optimized human sequences (A sequence, B sequence and C sequence) were obtained, wherein, these sequences specially optimized by the applicant are suitable for expression in Pichia pastoris, And does not contain the sequence of EcoRI, SalI, BglII and NotI restriction enzyme cutting sites.
[0029] A sequence
[0030] A sequence heavy chain variable region protein sequence (A-H-P sequence, SEQ ID NO: 2)
[0031] EVQLVESGGGLVKPGGSLRLSCAASGFTFSDYYMSWIRQAPGKGLEWVSYISSSGSTIY YASWVKGRFTISRDNAKNSLYLQXNSLRAEDTALYYCAKDIGYCTGGVCPQA
[0032] A sequence light chain variable region protein sequence (A-L-P sequence, SEQ ID NO: 3)
[0033] QFYADSAPLCVGVSGEDGNHLLHPQQWQHCQQLCAVVPTAPGQFPTTVIYEDHQRPSG IPDRFSASIDSSSSNSASLIISGLKTEDEADYYCQSYDTNNRVFGGGTKLTVLG
[0034] A sequence heavy chain variable regio...
Embodiment 4
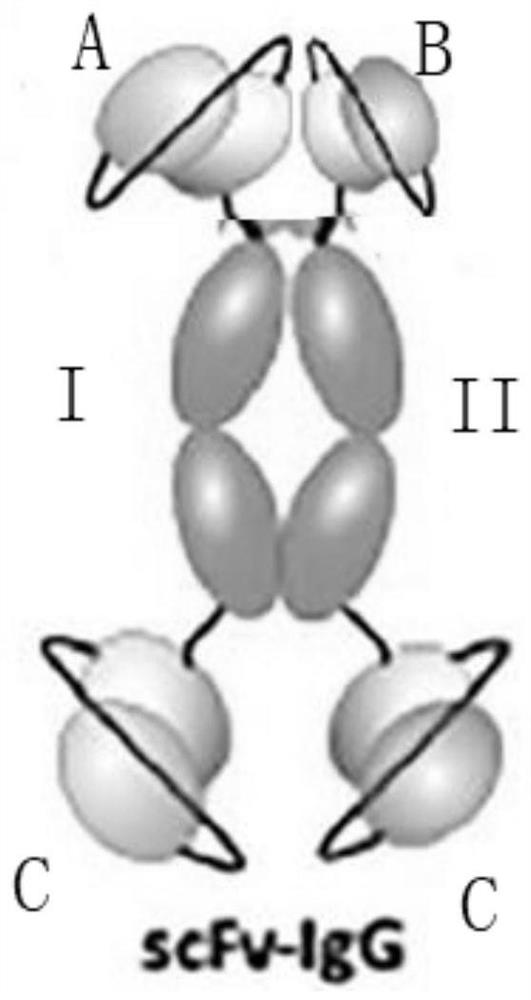
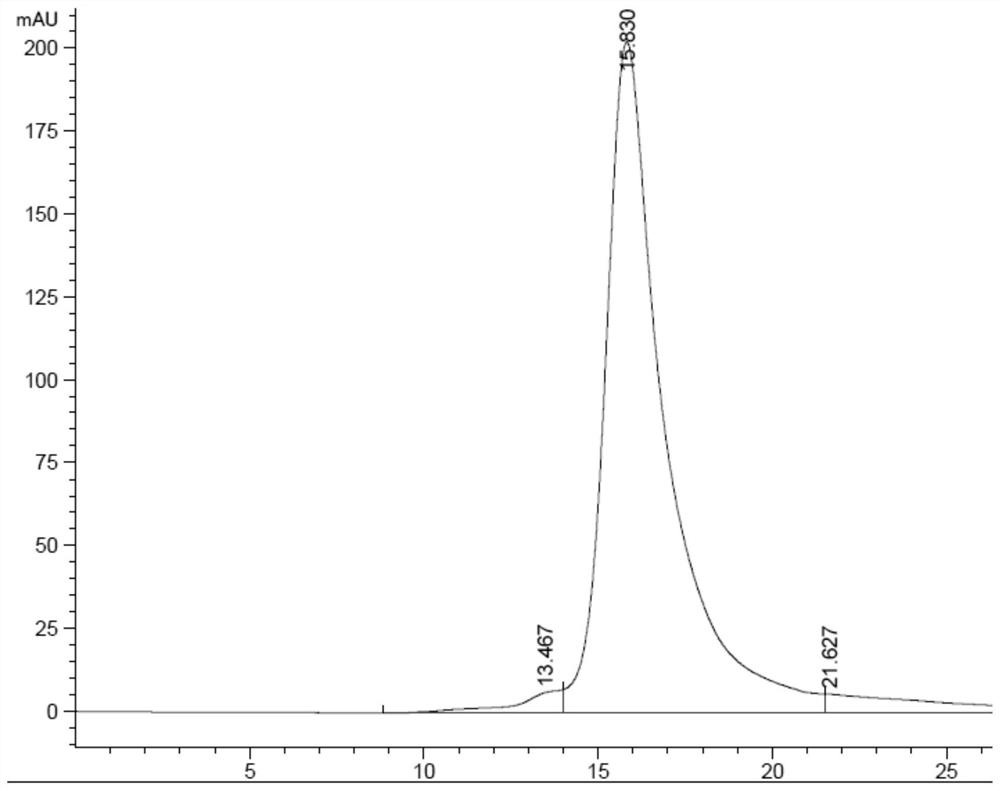
[0091] The measurement results showed that A, B and C had neutralizing and protective effects respectively, and their titers were 200IU / mg, 150IU / mg and 120IU / mg respectively. The combination has a synergistic neutralizing protective effect: ①The potency of group A+B is 350IU / mg; ②The potency of group B+C is 280IU / mg; ③The group has the best protective effect, and its titer is higher than the current horse anti-tetanus F(ab)2 titer 400-450IU / mg (because the molecular weight of horse anti-tetanus F(ab)2 is small) and human tetanus 200IU / mg of Influenza Immunoglobulin (TIG). Therefore, in the next experiments, the A+B+2C trivalent antibody was further expressed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com