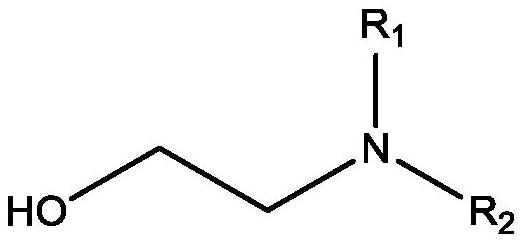
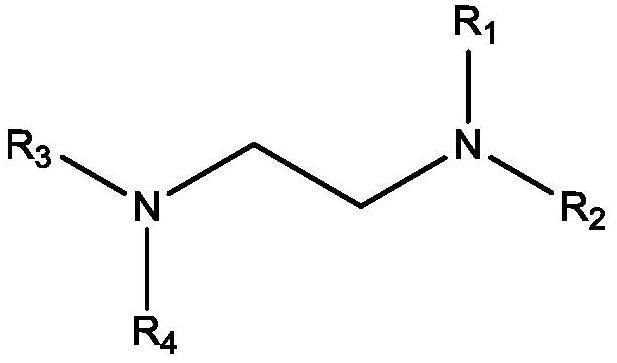
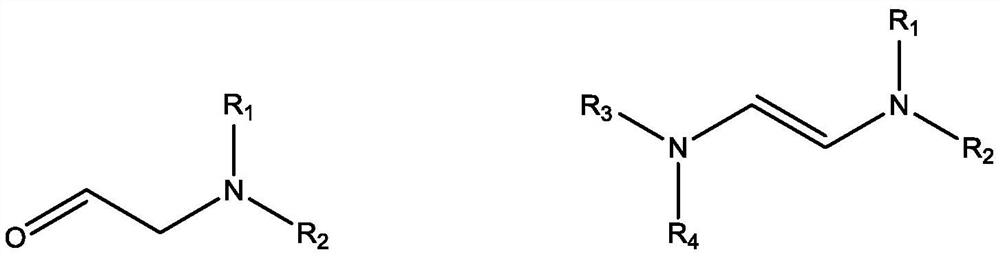
Reaction of glycolaldehyde
A glycolaldehyde, reaction technology, applied in the field of reaction of glycolaldehyde and aminating agent, can solve the problem of reducing the yield of the target product, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0142] The examples are to be understood as illustrating the method according to the invention. However, these examples should not be construed as limiting the scope of the invention.
[0143] Examples 1–6
[0144] In an electrically heated 50 ml autoclave (Hastelloy) with a mechanical magnetic stirrer was charged 0.5 g of commercially available dimer glycolaldehyde in a specific organic solvent (15 ml) and 200 μl of triethylene glycol dimethyl ether as an internal mark. Subsequently, the amount of catalyst specified in Table 1 was added. Dimethylamine was then metered in according to the molar ratios specified in Table 1 (dimethylamine:monomeric glycolaldehyde), the mixture was pressurized to 70 bar hydrogen and heated to 100°C. Stirring was carried out at 100°C for 1 hour under a specified pressure. The reaction product was filtered from the catalyst after 1 hour and analyzed by GC (carbon percentage, refers to the amount of carbon atoms of the substrate that can be dete...
example 7-11
[0151] An electrically heated 50 ml autoclave (Hastelloy) with a mechanical magnetic stirrer was charged with 1 g of commercially available dimer glycolaldehyde in a specific organic solvent (25 ml) and 400 μl of triglyme as an internal standard . Subsequently, the amount of catalyst specified in Table 2 was added. Ammonia was then metered in according to the molar ratios specified in Table 2 (ammonia:monomeric glycolaldehyde), the mixture was pressurized to 70 bar hydrogen and heated to 100°C. Stirring was carried out at 100°C for 1 hour under a specified pressure. The reaction product was filtered from the catalyst after 1 hour and analyzed by GC (carbon percentage, refers to the amount of carbon atoms of the substrate that can be detected as the final product, referenced to internal standard). The conversion was 100%, the difference from 100% mass balance was the unidentified minor component.
example 12-15
[0158] An electrically heated 50 ml autoclave (Hastelloy) with a mechanical magnetic stirrer was charged with 1 g of commercially available dimer glycolaldehyde in a specific organic solvent (25 ml) and 200 μl of triglyme as an internal standard . Subsequently, the amount of catalyst specified in Table 3 was added. Then, monomethylamine was metered in according to the molar ratio specified in Table 3 (monomethylamine:monomeric glycolaldehyde), the mixture was pressurized to 70 bar hydrogen and heated to 100°C. Stirring was carried out at 100°C for 1 hour under a specified pressure. The reaction product was filtered from the catalyst after 1 hour and analyzed by GC (carbon percentage, refers to the amount of carbon atoms of the substrate that can be detected as the final product, referenced to internal standard). The conversion was 100%, the difference from 100% mass balance was the unidentified minor component.
PUM
| Property | Measurement | Unit |
|---|---|---|
| relative permittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com