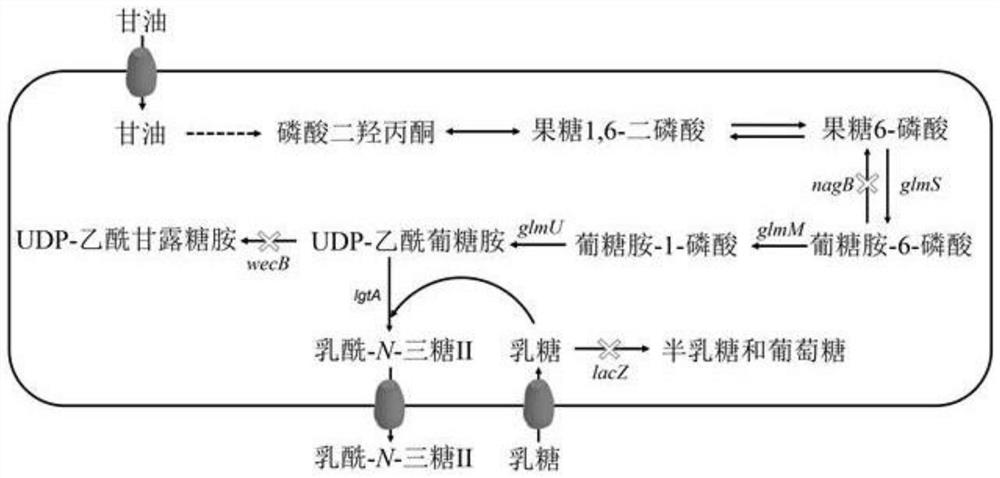
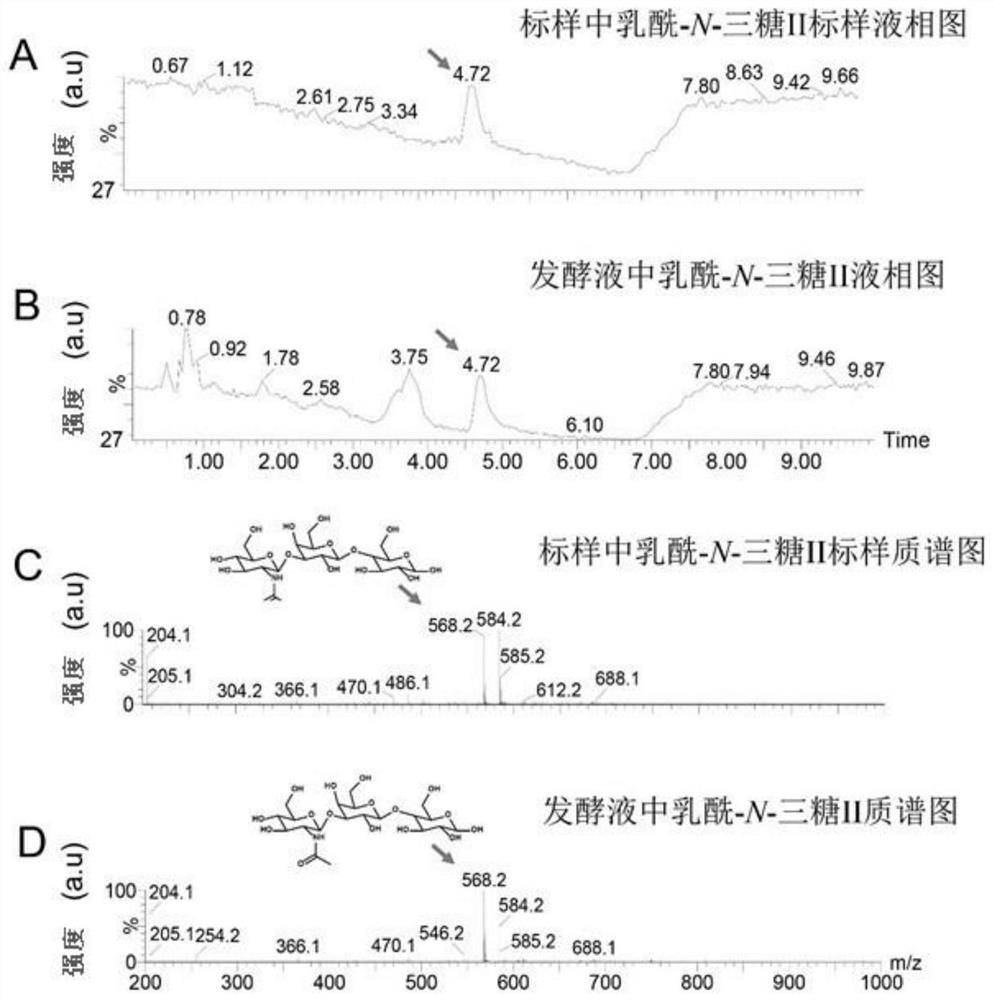
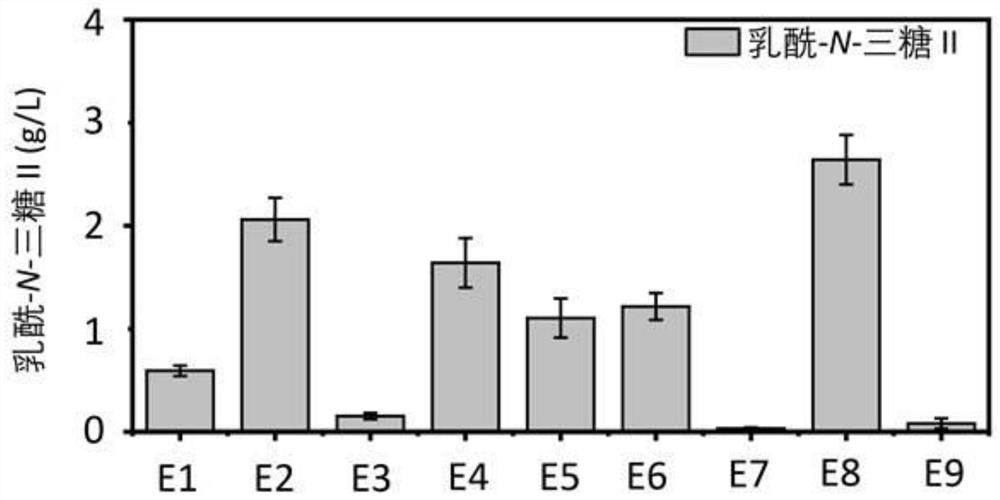
Genetically engineered bacteria capable of increasing yield of lactoyl-N-trisaccharide II and production method for genetically engineered bacteria
A technology encoding gene, acetylglucosamine, applied in microorganism-based methods, biochemical equipment and methods, glycosylases, etc., can solve the problem of low yield of lactoyl-N-trisaccharide II and inability to achieve industrialized large-scale production and other problems to achieve the effect of relieving metabolic stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] Preparation of competent E. coli: TAKARA kit.
[0042] Lactoyl-N-triose II fermentation process and detection:
[0043] LB liquid medium: peptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L.
[0044] LB solid medium: 10g / L peptone, 5g / L yeast extract powder, 10g / L sodium chloride, 15g / L agar powder.
[0045] Fermentation medium: 20g / L glucose, 13.5g / L potassium dihydrogen phosphate, 4.0g / L diammonium hydrogen phosphate, 1.7g / L citric acid, 1.4g / L magnesium sulfate heptahydrate and 10ml / L trace metal elements; Trace metal elements include: 10g / L ferrous sulfate, 2.25g / L zinc sulfate heptahydrate, 1.0g / L anhydrous copper sulfate, 0.35g / L manganese sulfate monohydrate, 0.23g / L sodium borate decahydrate, 0.11g / L ammonium molybdate, 2.0g / L calcium chloride dihydrate.
[0046] (1) Lactoyl-N-triose II fermentation process: Inoculate the constructed strain in LB liquid medium, 37°C, 200rpm, and culture overnight for 12h to obtain seed liquid, and insert the seed liquid...
Embodiment 1
[0048] Embodiment 1: Construction of recombinant vector
[0049] The specific steps of recombinant expression vector construction are as follows (see Table 1 for the primer sequences involved):
[0050] (1) Acquisition of glmM, glmU-glmS gene fragments: Using the genome of Escherichia coli K-12 (Escherichia coli) as a template, using glmM-F / glmM-R as primers, PCR amplifies the glmM gene fragments, and recovers DNA from the gel The fragment was connected to the first multiple cloning site (MCS1) of the vectors pRSFDuet-1, pETDuet-1 and pCDFDuet-1 through the seamless cloning kit (Nanjing Novizan Life Science and Technology Co., Ltd.); glmUS-R is the primer, the glmU-glmS gene cluster fragment is amplified by PCR, the DNA fragment is recovered from the gel, and the gene is connected to the second polyclonal of the vector pRSFDuet-1, pETDuet-1 and pCDFDuet-1 by kpnI single enzyme digestion site (MSC2), the final plasmids obtained are pRSF-glmM-glmU-glmS, pCDF-glmM-glmU-glmS and ...
Embodiment 2
[0054] Example 2: Replacement of the original ribosome binding site on the expression plasmid
[0055] In addition to expressing the ribosome binding site (RBS T7) of the plasmid itself, the present invention also selects two RBS (RBS 29 and RBS 31) of different strengths reported in the literature (see Table 2 for different RBS sequences). In this embodiment Select RBS 29 (represents strong regulation), RBS T7 (represents moderate regulation), and RBS 31 (represents weak regulation) to regulate the protein translation intensity of each target gene.
[0056] Table 2 RBS sequence
[0057]
[0058] Using the constructed plasmids pRSF-glmM-glmU-glmS and pET-lgtA as templates, use primers 29lgtA-F / R, 31lgtA-F / R, 29M-F / R, 31M-F / R, 29US-F / R , 31US-F / R, the corresponding fragments were obtained (see Table 3 for primer sequences), and the fragments were assembled using the One Step Cloning Kit (Vazyme) kit to obtain the corresponding recombinant plasmids (see Table 4 for plasmid i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com