Method capable of carrying out in-vitro off-target detection and sgRNA screening in batches
An off-target, batch technology, applied in the field of genetic engineering, to achieve the effect of high throughput, reliable experimental results and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
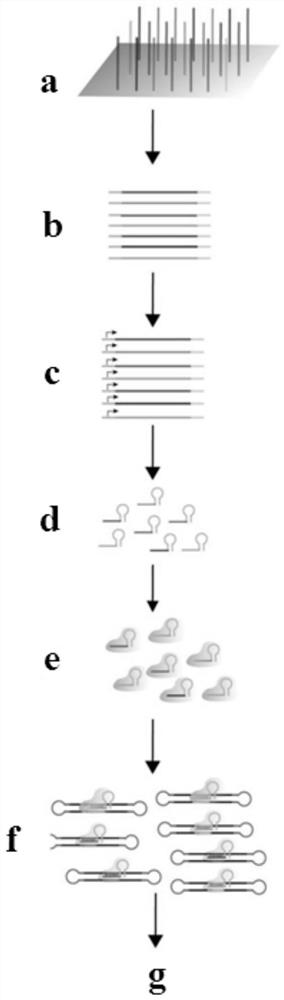
[0033] Specific Embodiment 1: In this embodiment, a method for in vitro off-target detection and sgRNA screening can be carried out in batches, which is completed according to the following steps:
[0034] 1. Collect 4×10 6 ~8×10 6 transfer the cells to a centrifuge tube, centrifuge, discard the medium, wash once with PBS, centrifuge again, discard the supernatant, and extract the cellular genomic DNA of the solid phase after centrifugation;
[0035] 2. Fragment the genomic DNA extracted in step 1 into a 300bp-700bp fragment, and then purify it with DNA purification magnetic beads;
[0036] 3. Perform end repair, add A tail, and add stem-loop structure linker 1 to the DNA purified in step 2, then treat it with exonuclease, and then treat it with ddNTP;
[0037] 4. Design and synthesize sgRNA oligo library, perform PCR amplification and in vitro transcription to obtain sgRNA pool;
[0038] 5. Use the Cas enzyme and the sgRNA pool obtained in step 4 to cut the ddNTP-treated D...
specific Embodiment approach 2
[0043] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is: in step 2, the instrument Bioruptor is used to interrupt DNA, parameters: DNA 50ng / μL; volume 100 μL; 15sON-90sOFF; 6-8 cycles; interruption The final DNA was purified with DNA purification magnetic beads, and the elution volume was 37 μL.
[0044] Other steps are the same as in the first embodiment.
specific Embodiment approach 3
[0045] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that in step 3, a kit is used to repair DNA ends, add A tails, and add stem-loop structure adapter 1, wherein the DNA ends are repaired, A tails are added Concrete reaction system and reaction procedure are as follows:
[0046] Kit: ABclonal Rapid DNA Library Construction Kit
[0047]
[0048] Reaction procedure: 20°C for 30 minutes; 65°C for 30 minutes to obtain the end repair mixture;
[0049] The specific reaction system and reaction procedure of adding stem-loop structure linker 1 are as follows:
[0050]
[0051] Reaction program: 1h at 22°C, then 1×DNA purification magnetic beads purification, elution volume 30 μL.
[0052] Other steps are the same as those in Embodiment 1 or 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com