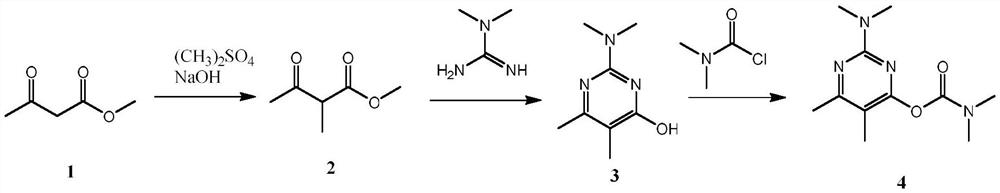
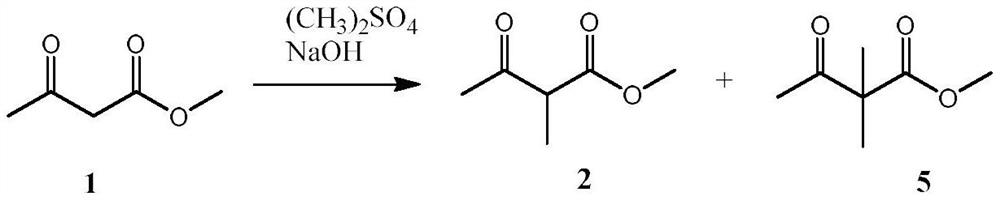
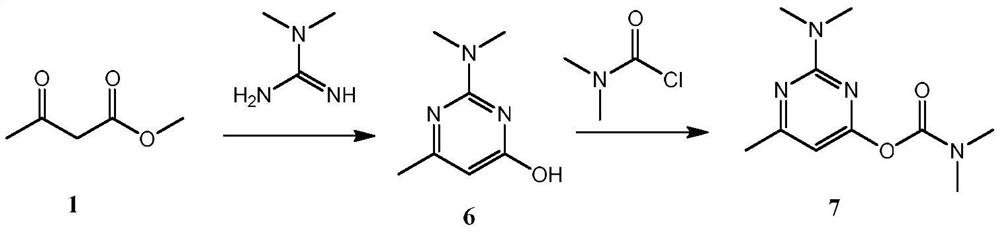
A kind of synthetic method of pirimicarb intermediate 2-methyl acetoacetate methyl ester
A technology of methyl acetoacetate and a synthesis method, which is applied in the synthesis field of pirimicarb intermediate 2-methyl acetoacetate, can solve the problems of low yield, side reactions and the like, and achieves high product yield , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Synthesis of methyl 2-acetyl acrylate:
[0042] Under nitrogen protection, add 10.0g methyl acrylate (98%, 114mmol), 10.0g acetyl chloride (98%, 125mmol), 40ml ether and 0.05g 4-methoxyl-phenol in a three-necked flask, add dropwise 12.92g under a water bath Triethylamine (98%, 125mmol), keep the reaction temperature at 20-25°C, keep it warm for 4 hours after the dropwise addition, add 40ml of water solution, wash the organic phase with 30ml of water three times, dry over anhydrous magnesium sulfate, and concentrate under vacuum to obtain 16.5g pale yellow liquid.
[0043] GC-MS(EI): 128.1, 1 H NMR: (400MHz, CDCl 3 ):ppmδH 5.80(s,1H), 5.77(s,1H), 3.75(s,3H) 2.30(s,3H).
[0044] (2) Synthesis of 2-methyl acetoacetate:
[0045] Dissolve 16.5g of the above product in 60ml of ethanol in a hydrogenation kettle, add 1.0g of 5% palladium-carbon catalyst (5g of metal palladium loaded on 95g of activated carbon), replace the air and feed hydrogen to 0.05MPa, and react at ...
Embodiment 2
[0048] (1) Synthesis of methyl 2-acetyl acrylate:
[0049] Under nitrogen protection, add 10.0g methyl acrylate (98%, 114mmol), 10.0g acetyl chloride (98%, 125mmol), 40ml methyl tert-butyl ether and 0.05g 2-tert-butyl-4-methane Base phenol, add 16.5g DIPEA (98%, 125mmol) dropwise in a water bath, keep the reaction temperature at 20-25°C, keep warm for 4 hours after the dropwise addition, add 40ml of water, wash the organic phase three times with 30ml of water, and dry over anhydrous magnesium sulfate , concentrated under reduced pressure in vacuo to obtain 16.8 g of light yellow liquid.
[0050] (2) Synthesis of 2-methyl acetoacetate:
[0051] Dissolve 16.8g of the above product in 60ml of ethanol in a hydrogenation kettle, add 1.0g of 5% palladium-carbon catalyst (5g of metal palladium supported on 95g of activated carbon), replace the air and feed hydrogen to 0.05MPa, and react at room temperature for 5 Hours, after the reaction was completed, the catalyst was removed by fil...
Embodiment 3
[0053] (1) Synthesis of methyl 2-acetyl acrylate:
[0054] Under nitrogen protection, add 10.0g methyl acrylate (98%, 114mmol), 10.0g acetyl chloride (98%, 125mmol), 30ml 2-methyltetrahydrofuran and 0.05g 2,6-di-tert-butyl-4 -Methyl phenol, add DABCO's 2-methyltetrahydrofuran solution (14.3g DABCO (98%, 125mmol), 20ml 2-methyltetrahydrofuran) dropwise under a water bath, keep the reaction temperature at 20-25°C, and keep the temperature for 4 40ml water solution was added, the organic phase was washed three times with 30ml water, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure in vacuo to obtain 16.7g light yellow liquid.
[0055] (2) Synthesis of 2-methyl acetoacetate:
[0056] Dissolve 16.7g of the above product in 60ml of ethanol in a hydrogenation kettle, add 1.0g of 5% palladium-carbon catalyst (5g of metal palladium supported on 95g of gac), replace the air and feed hydrogen to 0.05MPa, and react at room temperature for 5 Hours, after th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com