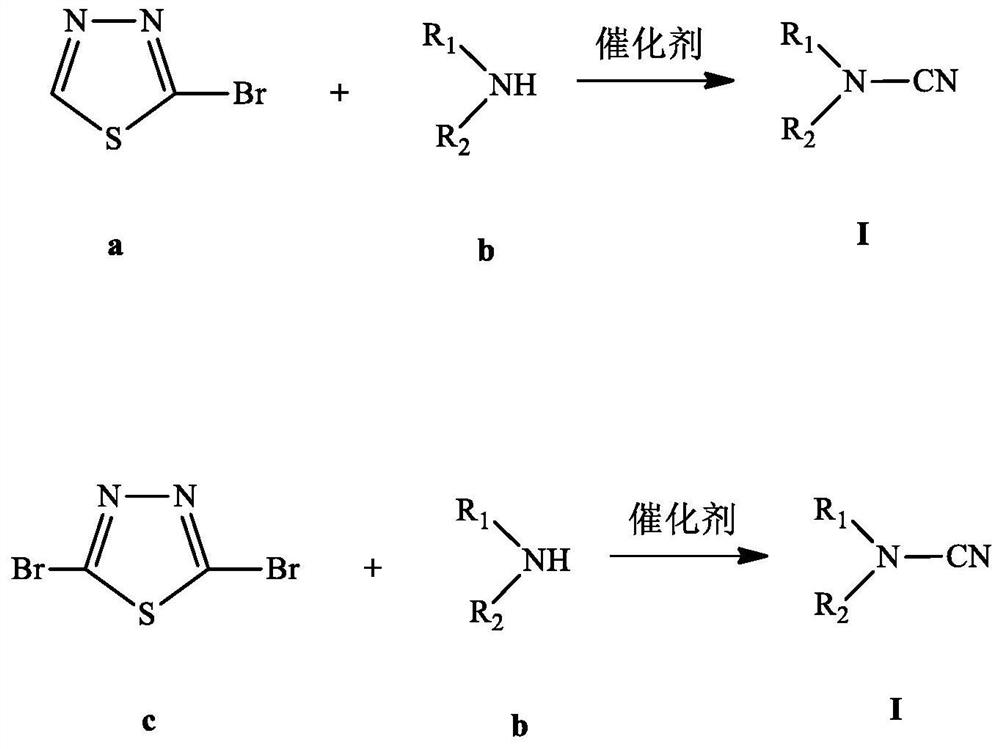
Preparation method of cyano tertiary amine
A technology based on tertiary amines and alkyl groups, applied in the field of preparation of cyano tertiary amines, can solve the problems of high toxicity of cyanogen halide, limited wide application, lack of versatility, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067]
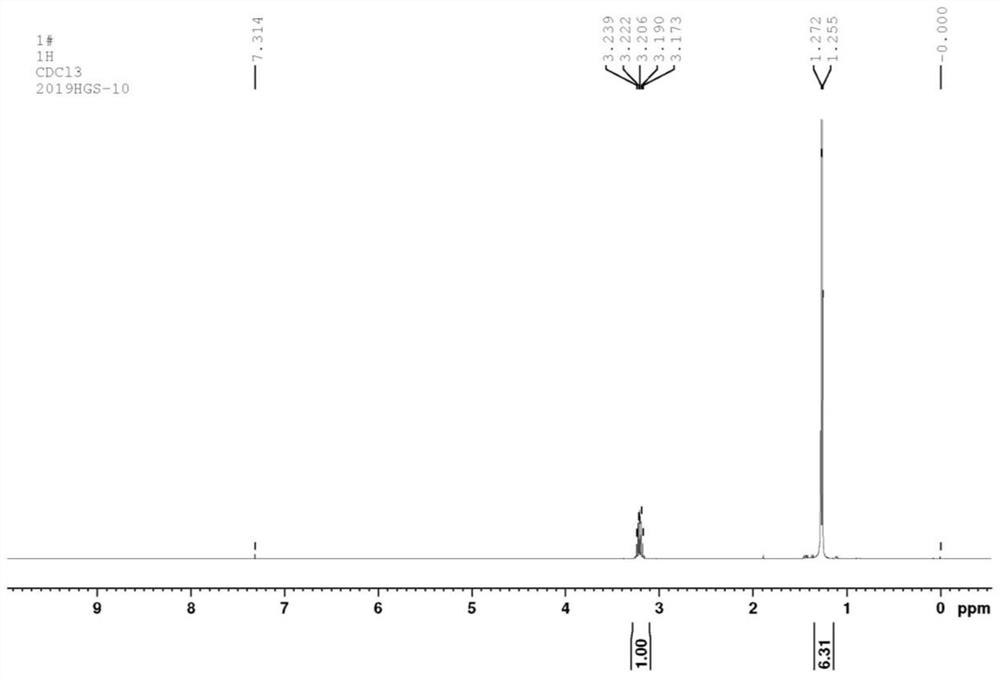
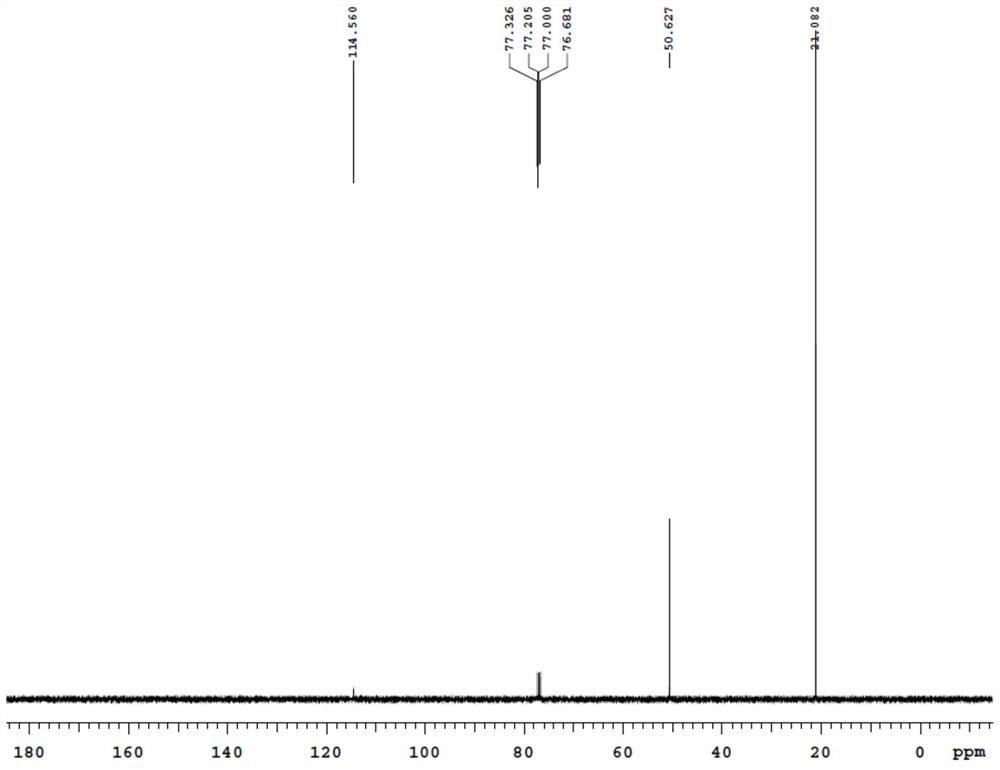
[0068] Add 16.5 grams (0.1mol) of 2-bromo-1,3,4-thiadiazole, 60.72 grams (0.6mol) of diisopropylamine, 0.15 grams of copper powder and 1.64 grams of cuprous bromide to 250 ml In a round-bottomed flask, heat and reflux for 2 hours, after naturally cooling to room temperature, suction filtration, the filtrate is removed unreacted diisopropylamine by a rotary evaporator, and 10.66 grams of a light yellow liquid is obtained by distillation under reduced pressure, with a yield of 84.5%. ; IR (KBr tablet, cm -1 ):2976.16, 2933.73, 2877.79, 2196.92, 1523.76, 1463.97, 1386.82, 1371.39, 1334.74, 1242.16, 1184.29, 1130.29, 1101.35, 1053.13, 893.04; 1 H NMR (400MHz, CDCl 3 )δ (ppm): 3.17-3.23 (m, 2H), 1.25-1.27 (d, 12H, J=6.8Hz); 13 C NMR (100MHz, CDCl 3 )δ (ppm): 114.56, 50.62, 21.08. MS(m / z) / (M + ):126[M + ](55), 111(25), 69(100).
Embodiment 2
[0070]
[0071] 16.5 grams (0.1mol) of 2-bromo-1,3,4-thiadiazole, 77.52 grams (0.6mol) of di-n-butylamine, 0.15 grams of copper powder and 1.64 grams of cuprous bromide were added to 250 In a round-bottomed flask of 1 ml, heat and reflux for 2 hours, after naturally cooling to room temperature, suction filtration, the filtrate removes unreacted di-n-butylamine through a rotary evaporator, and obtains 13.26 grams of a light yellow liquid through vacuum distillation. The yield is 85.9%; 1 H NMR (400MHz, CDCl 3 )δ(ppm):2.96-3.00(t,4H),1.59-1.63(m,4H),1.36-1.44(m,4H),0.93-0.95(t,6H); 13 C NMR (100MHz, CDCl 3 )δ (ppm): 110.5, 51.20, 29.67, 19.68, 13.63. MS(m / z) / (M + ):154[M + ](20), 125(10), 111(35), 84(10), 69(100), 56(60), 44(25).
Embodiment 3
[0073]
[0074] 16.5 grams (0.1mol) of 2-bromo-1,3,4-thiadiazole, 17.03 grams (0.2mol) of piperidine, 0.15 grams of copper powder, 1.64 grams of cuprous bromide and 100 milliliters of toluene Join in the round-bottomed flask of 250 milliliters, heat and reflux for 2 hours, after naturally cooling to room temperature, suction filtration, filtrate removes toluene and unreacted piperidine through rotary evaporator, obtains 6.82 grams of light yellow liquid through decompression distillation, The yield is 61.9%; 1 H NMR (400MHz, CDCl 3 )δ (ppm): 3.16-3.19 (t, 4H), 1.62-1.68 (m, 4H), 1.54-1.60 (m, 2H); 13 C NMR (100MHz, CDCl 3 )δ (ppm): 118.27, 49.83, 24.23, 22.64. MS(m / z) / (M + ):110[M + ](75), 82(10), 69(45), 56(45), 42(100), 28(20).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com