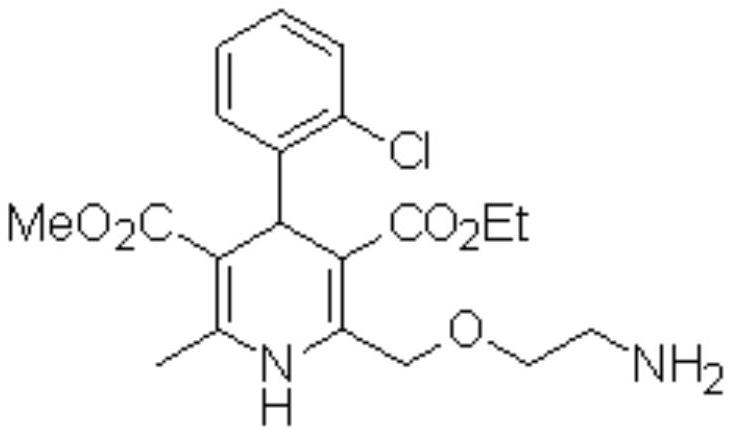
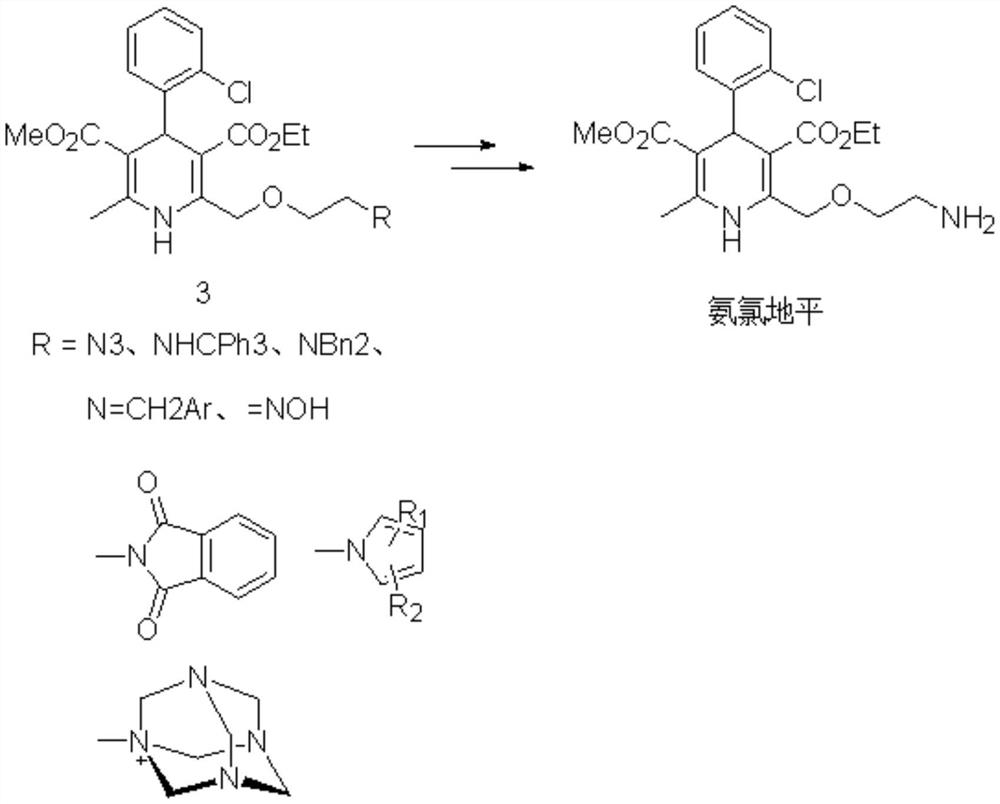
Preparation method of amlodipine base
A technology of amlodipine base and chlorophenyl, which is applied in the field of preparation of amlodipine base, can solve the problems of unstable intermediates, high equipment requirements, poor safety, etc., and achieve a simple route, reduced production cost, and low price. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
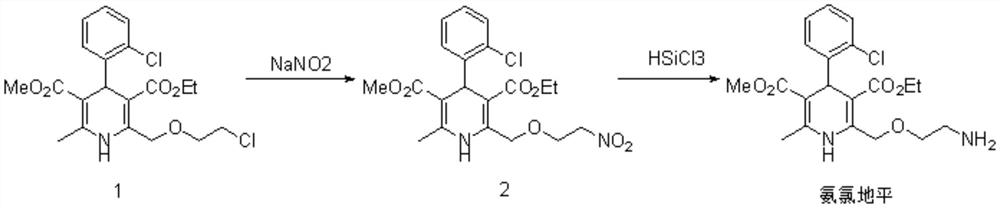
[0024] Add 6-methyl-2-(2-chloroethoxy)methyl-4-(2-chlorophenyl)-1,4-dihydro-3,5-pyridinedicarboxylic acid methyl ethyl to the 10L reaction flask 1.0 mol of ester, 0.1 mol of potassium iodide and 5L of acetone were dissolved by stirring, then 1.5 mol of sodium nitrite was added, the reaction liquid was heated to 50°C and stirred for 6 hours; suction filtered while it was hot, the filtrate was concentrated under reduced pressure, and the obtained residue was recrystallized with water to obtain Yellow solid product, yield 81.1%.
[0025] Under the protection of nitrogen, ethyl 6-methyl-2-(2-nitroethoxy)methyl-4-(2-chlorophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate 0.5mol, dissolved in 5L of dichloromethane, added 2.5mol of triethylamine, cooled to -5°C with ice-brine, slowly pressed 1.5mol of trichlorosilane from the cylinder, and then the reaction solution was slowly raised to 25°C and stirred for 24 Hour. Slowly add 3L saturated aqueous solution of sodium bicarbonate dropwis...
Embodiment 2
[0027] Add 6-methyl-2-(2-chloroethoxy)methyl-4-(2-chlorophenyl)-1,4-dihydro-3,5-pyridinedicarboxylic acid methyl ethyl to the 10L reaction flask Add 1.0mol of ester, 0.01mol of tetrabutylammonium iodide and 5L of acetonitrile and acetone mixed solvent, stir to completely dissolve, add 1mol of sodium nitrite, heat the reaction solution to 60°C and stir for 6 hours; filter while hot, and concentrate the filtrate under reduced pressure , the resulting residue was recrystallized from water to obtain a yellow solid product with a yield of 80.7%.
[0028] Under the protection of nitrogen, ethyl 6-methyl-2-(2-nitroethoxy)methyl-4-(2-chlorophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate Dissolve 1mol in 5L of acetonitrile, add 3mol of diisopropylethylamine, cool down to -5°C in ice brine, slowly press in 4mol of trichlorosilane from the cylinder, then slowly raise the reaction solution to 25°C and stir for 24 hours . Slowly add 3L saturated aqueous solution of sodium bicarbonate dropw...
Embodiment 3
[0030] Add 6-methyl-2-(2-chloroethoxy)methyl-4-(2-chlorophenyl)-1,4-dihydro-3,5-pyridinedicarboxylic acid methyl ethyl to the 10L reaction flask Add 1.0 mol of ester, 0.2 mol of sodium iodide and 5 L of N, N-dimethylformamide, stir to completely dissolve, add 1.8 mol of sodium nitrite, heat the reaction solution to 40°C and stir for 6 hours; suction filter while hot, and depressurize the filtrate After concentration, the resulting residue was recrystallized from water to obtain a yellow solid product with a yield of 80.9%.
[0031] Under the protection of nitrogen, ethyl 6-methyl-2-(2-nitroethoxy)methyl-4-(2-chlorophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate 1mol, dissolved in 5L tetrahydrofuran, add 7mol of diisopropylethylamine, cool down to -5°C in ice brine, slowly press 2mol of trichlorosilane from the cylinder, then slowly raise the reaction solution to 25°C and stir for 24 hours . Slowly add 3L saturated aqueous solution of sodium bicarbonate dropwise, stir for 2 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com