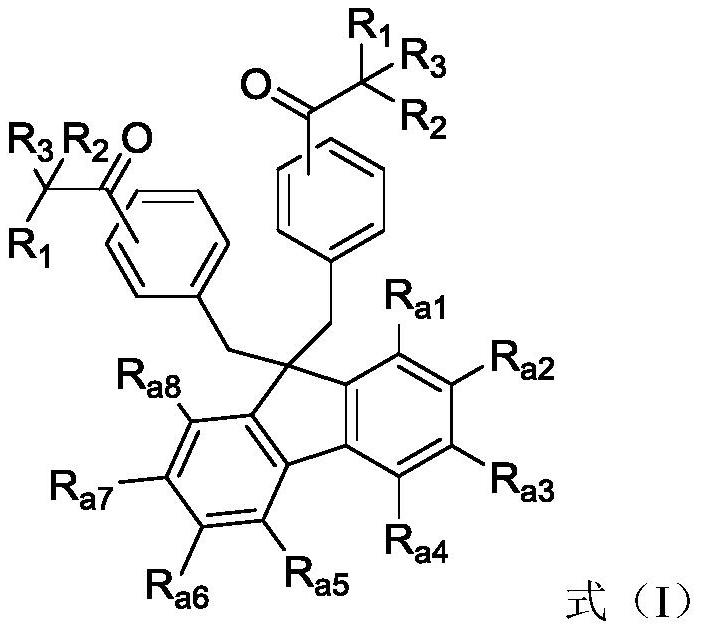
Fluorene photoinitiator, preparation method thereof, photocuring composition containing fluorene photoinitiator and application of fluorene photoinitiator in field of photocuring
A photoinitiator and compound technology, applied in the field of photocuring, can solve the problems of high initiation efficiency, photoinitiator cannot maintain zero mobility, high yellowing resistance, etc., to achieve improved absorption efficiency, good photoinitiation efficiency, durability Excellent effect of yellowness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Another aspect of the present application also provides a kind of preparation method of above-mentioned fluorene photoinitiator, and this preparation method comprises:
[0026] S1, in the presence of the first organic solvent, intermediate a undergoes a substitution reaction with a halogenated compound to generate intermediate b, and the synthetic route is as follows:
[0027]
[0028] Among them, X 1 is a halogen atom;
[0029] S2, in the presence of a second organic solvent, the Friedel-Crafts reaction of the intermediate b and the halogenated alkanoyl compound is carried out to obtain the intermediate c, and the synthetic route is as follows:
[0030]
[0031] Among them, X 2 and X' are independently selected from halogen atoms;
[0032] S3, hydrolyzing the intermediate c with water to obtain a fluorene-based photoinitiator, or performing a dehalogenation reaction between the intermediate c and a compound containing a non-hydroxyl photoactive group to obtain...
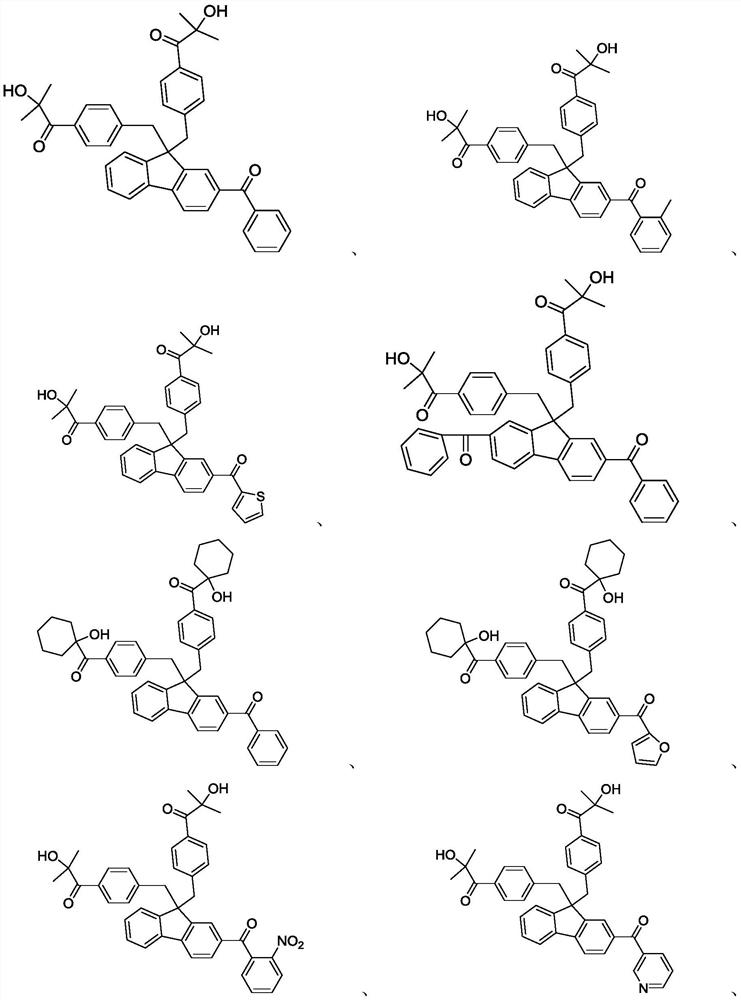
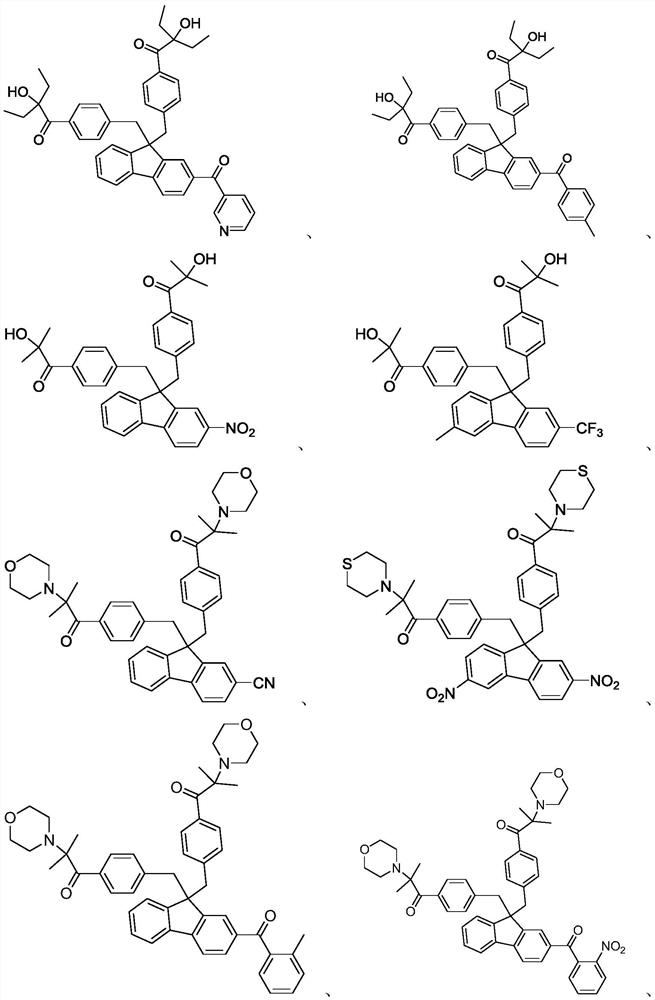
Embodiment 1 to 12
[0049] Step (1): Preparation of 9,9-dibenzylphenyl ketone fluorene (a1)
[0050]
[0051] Add 150mL of dichloromethane, 27g of phenyl ketone fluorene, 28.05g of benzyl chloride and 0.54g of tetrabutylammonium bromide into a 250mL four-necked flask, slowly add 40g of 50% sodium hydroxide solution dropwise, and control the temperature of the system at 30 Below ℃, the addition is completed in 15 minutes, the reaction is heated in a water bath at 50 ℃, the nitrogen protection is stopped, the system is kept warm for 8 hours, and the reaction is stopped. After the temperature of the system dropped to room temperature, 60 g of water was added, the system was separated into layers, and the organic layer was separated. The organic layer was washed 3 times with 200 g of water until neutral. Pour the organic phase into a 250mL four-port screen, distill off the solvent, add 54g of methanol, stir and crystallize in an ice-water bath for 1h, filter, rinse the filter cake with a little me...
Embodiment 13-24
[0068] Referring to the synthesis methods of intermediates a1, b2, and c3 in Examples 1-12, compounds 13-24 were synthesized by different preparation methods.
[0069] Synthesis of compound 13:
[0070]
[0071] Add 50g of intermediate a3 and 200g of morpholine to a 250mL four-neck flask, heat and reflux at 100°C for 6h, track the liquid phase until the reaction is complete, then pour the reaction solution into water and stir, and a beige solid precipitates, suction filters, washes with water, and reconstitutes with methanol Crystallization gave 51 g of a white solid, namely compound 11, with a yield of 88.5% and a purity of 98.01%.
[0072] The structure of the product was confirmed by H NMR and mass spectrometry:
[0073] 1 H-NMR (CDCl 3 , 400MHz): δ8.10–7.94(m,5H),7.87–7.78(m,2H),7.70–7.62(m,2H),7.55(t,J=8.3Hz,3H),7.48-7.37(m ,3H),7.33-7.27(m,1H),6.65(d,J=8.3Hz,4H),3.61–3.43(m,12H),2.48–2.35(m,8H),1.20(s,12H). .
[0074] MS (m / z): 761 (M+1) + .
[0075] Referring...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com