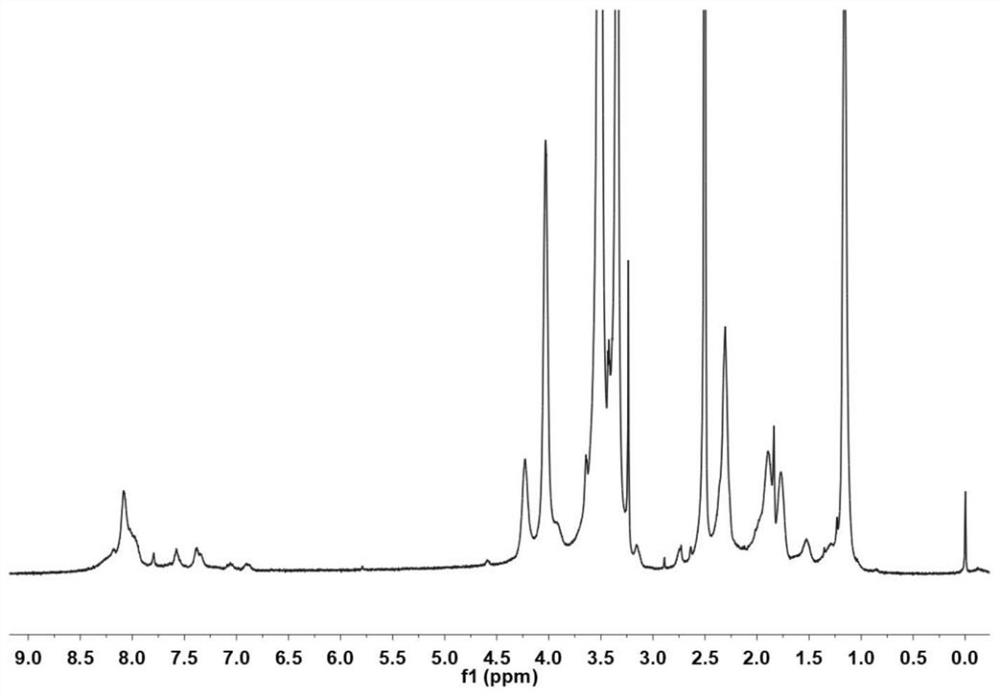
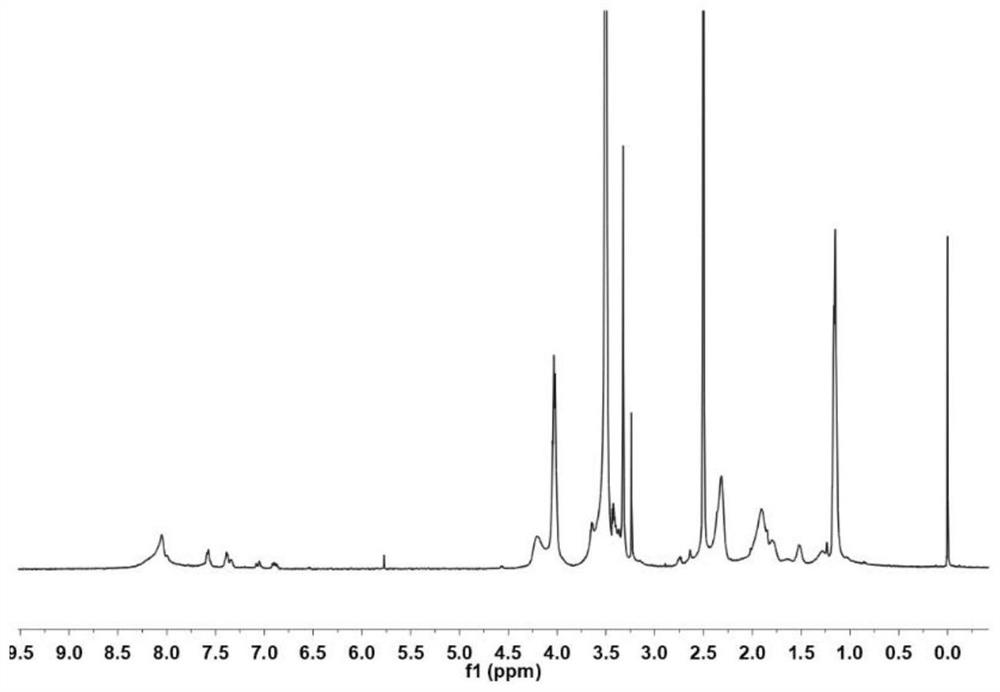
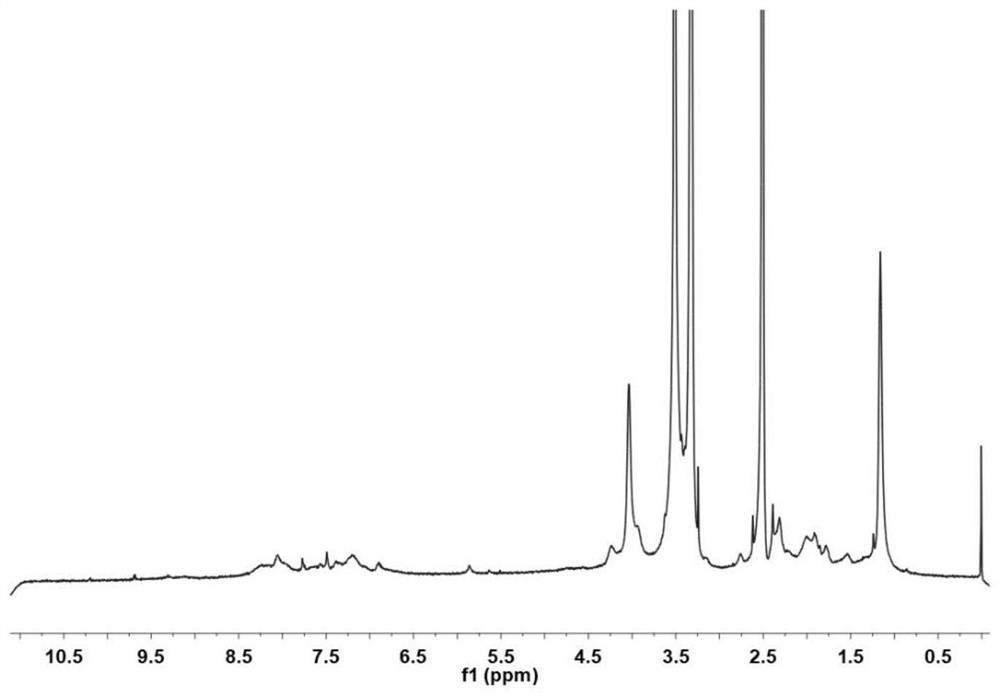
Cinnamyl aldehyde modified polyethylene glycol-polyamino acid block copolymer, preparation method thereof, and hydrogel
A technology of block copolymer and polyethylene glycol, which is applied in the field of polyamino acids, can solve the problems of restricting the application of temperature-sensitive injectable hydrogels, lack of drug properties or biological activity, etc., so as to improve drug delivery efficiency and prolong drug delivery. Drug time, the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention further provides a polyethylene glycol modification of the above cinnamaldehyde - preparation of polyamino acid block copolymer characterized by comprising the steps of:
[0050] The polyethylene glycol compound, γ- -N- ethyl -L- glutamate and the carboxylic anhydride ε- benzyloxycarbonyl -L- lysine -N- mixed acid anhydride within acid polymerization reaction to obtain a reaction product;
[0051] The reaction product was sequentially passes terminated acetyl, hydrobromic acid and the deprotection treatment cinnamaldehyde grafting reaction to give cinnamic aldehyde-modified polyethylene glycol - poly-amino acid block copolymer;
[0052] Amino-terminated polyethylene glycol monomethyl ether or polyethylene glycol structure of formula V as shown in the polyethylene glycol compound selected from a structure of the terminal amino group of Formula IV;
[0053]
[0054] Wherein, m is the degree of polymerization, 10≤m≤227
[0055] n is the degree of polyme...
Embodiment 1
[0098] Polyethylene glycol monomethyl ether in Example 1 (terminal amino group of: γ- -N- ethyl -L- glutamate anhydride within acid: ε- benzyloxycarbonyl -L- lysine -N- the carboxylic acid anhydride monomer = 1: 5: 2)
[0099] To a dry reaction bottle was added 2g, number average molecular weight of 550 amino end of polyethylene glycol monomethyl ether. And 100mL of dry toluene under azeotropic removal of water 130 ℃ 2h, drained residual toluene under reduced pressure; the resulting solid was dissolved in 50mL dried after addition of water, N, N- dimethyl formamide, to give a first solution ;
[0100] The said 3.7gγ- -N- ethyl -L- glutamate and the carboxylic anhydride group 2.22gε- benzyloxycarbonyl -L- lysine -N- acid anhydride was dissolved in 50mL the dewatering drying after the N, N- dimethylformamide to give a second solution;
[0101] Protection under a nitrogen atmosphere, mixing the first solution with the second solution and stirred for 24 hours at 45 deg.] C; After comp...
Embodiment 2
[0105] Polyethylene glycol monomethyl ether Example 2 (terminal amino group of: γ- -L- glutamate ethyl ester -N- acid anhydride: ε- benzyloxycarbonyl -L- lysine -N- the carboxylic acid anhydride monomer = 1: 8: 2)
[0106] To a dry reaction bottle was added 2g, number average molecular weight of 550 amino end of polyethylene glycol monomethyl ether. And 100mL of dry toluene under azeotropic removal of water 130 ℃ 2h, drained residual toluene under reduced pressure; the resulting solid was dissolved in 50mL dried after addition of water, N, N- dimethyl formamide, to give a first solution ;
[0107] The said 5.92gγ- -N- ethyl -L- glutamate and the carboxylic anhydride group 2.22gε- benzyloxycarbonyl -L- lysine -N- acid anhydride was dissolved in 50mL the dewatering drying after the N, N- dimethylformamide to give a second solution;
[0108] Protection under a nitrogen atmosphere, mixing the first solution with the second solution and stirred for 24 hours at 45 deg.] C; After complet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com