Biological enzyme catalysis synthesis method for 6-bromoindanone
A synthesis method and technology of bromindanone are applied in the field of preparing medicines by using enzyme mutation technology, and the effects of high product rate, high yield and high purity are achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of mutants of SEQ ID NO.1: mutant library construction and high-throughput screening methods:
[0034] Construction of mutant library:
[0035] In order to improve the activity of transpeptidase, using the recombinant expression vector PET-28a(+)-BS as the DNA template, a random mutant library was constructed by error-prone PCR, and the concentration of Mg2+ and Mn2+ in the error-prone PCR reaction system was adjusted And the concentration of dCTP and dTTP oligonucleotides, so that the base mismatch rate of the mutant library is 10 per thousand, that is, to ensure that a mutant has 2 to 6 amino acid mutations. The specific process of constructing the mutant library is as follows. Error-prone PCR reaction system and conditions:
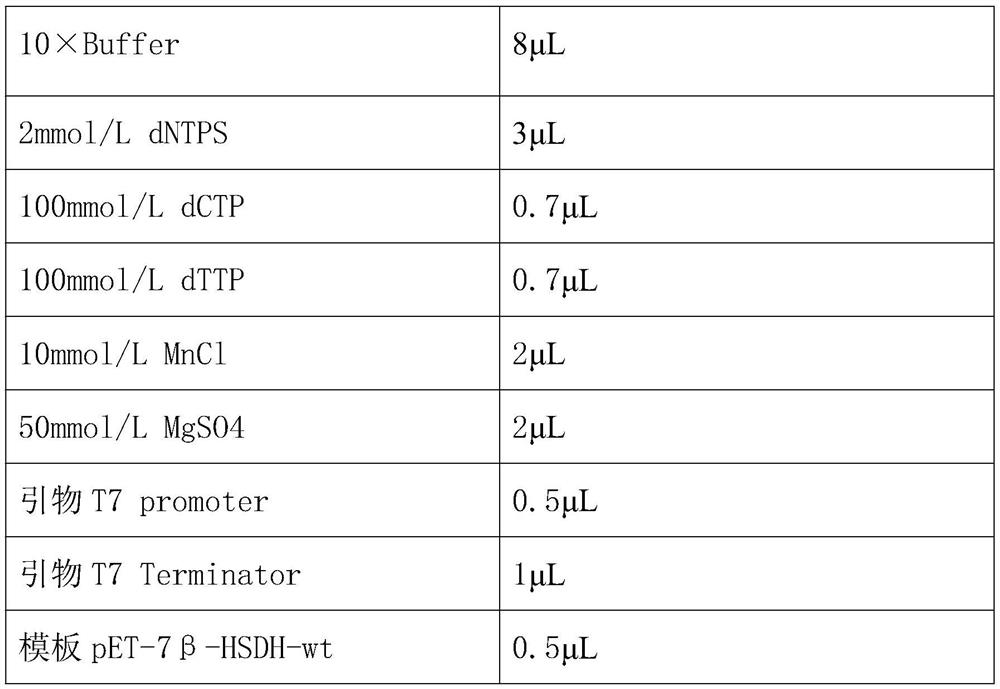
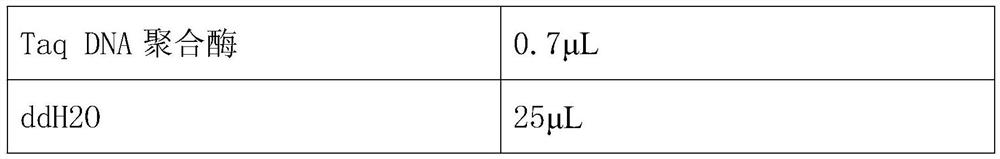
[0036] Error-prone PCR reaction system:
[0037] 10×Buffer 8μL 2mmol / L dNTPS 3μL 100mmol / L dCTP 0.7μL 100mmol / L dTTP 0.7μL 10mmol / L MnCl 2μL 50mmol / L MgSO4 2μL Primer T7 promoter ...
Embodiment 2
[0043] Add 229g of p-bromophenylpropionic acid (1.0mol) into 1000ml of methanol, stir to fully dissolve, then add 200ml of 5mM potassium dihydrogen phosphate buffer solution (pH=2.0), 0.95KU of mutated transpeptidase, room temperature Stir under low pressure for 20 hours, evaporate the organic solvent to dryness, filter to obtain the crude product, and then recrystallize with methanol / water to obtain 197.50 g of fine 6-bromoindanone, with a conversion rate of 93.83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com