A kind of green production technology of iodate
A technology of green production and iodate, which is applied in the field of green production technology of iodate, and can solve problems such as environmental pollution, environmental pollution, safety accidents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of potassium iodate:
[0043]Add 3600mL of deionized water to the 5000mL reaction kettle, put in 632g of lithium hydroxide (≥56.5%), 1910g of iodine, and 12.08g of potassium dichromate, take samples to detect the concentration and pH of lithium ions and potassium dichromate, and add an appropriate amount according to the test results. Water, lithium hydroxide, iodine, potassium dichromate, adjust the final lithium ion mass concentration to 1.7%, add potassium dichromate to adjust the potassium dichromate mass concentration to 0.2%, adjust the pH to 10.1, configure 3 batches of electrolysis according to this ratio The liquid is transferred to the electrolyte storage tank for backup.
[0044] The configured electrolyte part is put into the electrolysis device, the temperature is raised to 80°C, the cycle is turned on, the voltage is set to 3.8V, and the electrolysis is carried out until the mass concentration of iodate is 18%. The electrolysis completion liqu...
Embodiment 2
[0050] The preparation of potassium iodate (mother liquor is applied mechanically):
[0051] Get 4000mL of metathesis mother liquor obtained in embodiment 1, detect lithium ion, potassium ion, potassium dichromate concentration, pH value, add appropriate amount of water, lithium hydroxide and iodine fine-tuning according to the detection result, make the lithium ion mass concentration in the solution be 0.8 %, potassium ion mass concentration ≤ 1.3%, potassium dichromate mass concentration 0.2%, adjust PH to 9.6, configure 3 batches and transfer to electrolyte storage tank for standby.
[0052] Continuously add the above electrolyte in the 18% iodate-containing electrolysis circulation system stabilized in Example 1, control the electrolysis cathode and anode voltage 3.8V, and continuously extract the electrolysis completion solution from the discharge port, through the electrolyte feed valve, The flow rate was adjusted, the mass concentration of iodate in the extracted electr...
Embodiment 3
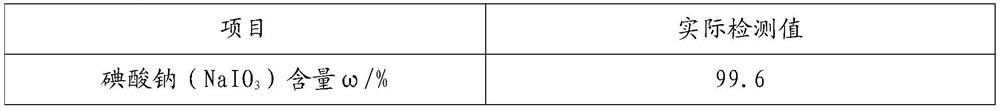
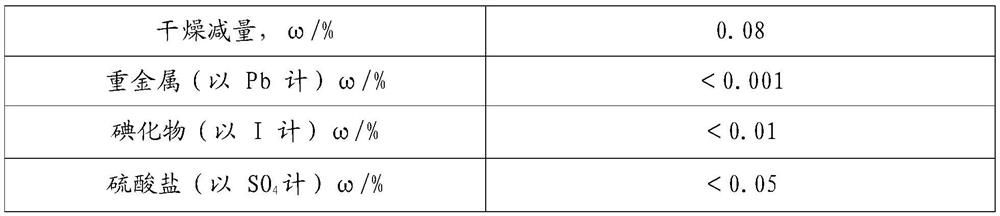
[0058] Preparation of sodium iodate:
[0059] Add 3600mL of deionized water to the 5000mL reaction kettle, put in lithium hydroxide and iodine, adjust the lithium ion mass concentration to 1.7%, add sodium dichromate to adjust the sodium dichromate mass concentration to 0.15%, adjust the pH to 10.6, according to this ratio Configure 4 batches of electrolyte and transfer it to the electrolyte storage tank for backup.
[0060] The configured electrolyte part is put into the electrolysis device, the temperature is raised to 80°C, the cycle is turned on, the voltage is set to 3.7V, and electrolyzed to 16% of the iodate mass concentration, and the electrolyte is continuously added to the electrolysis device, and continuously collected from the discharge port. The finished electrolysis solution is discharged, the flow rate is adjusted through the electrolyte feeding valve, the mass concentration of iodate in the extracted electrolysis finished solution is controlled to be 16%, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com