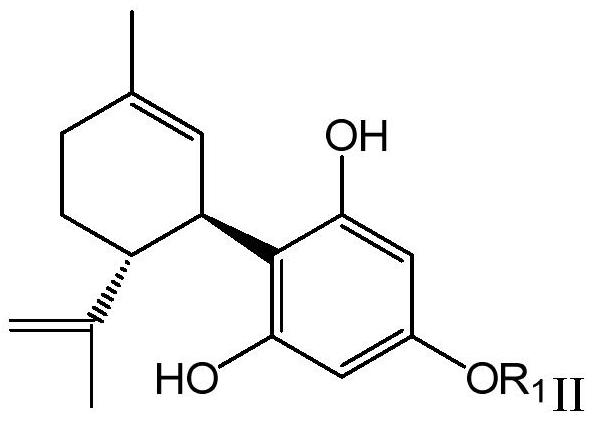
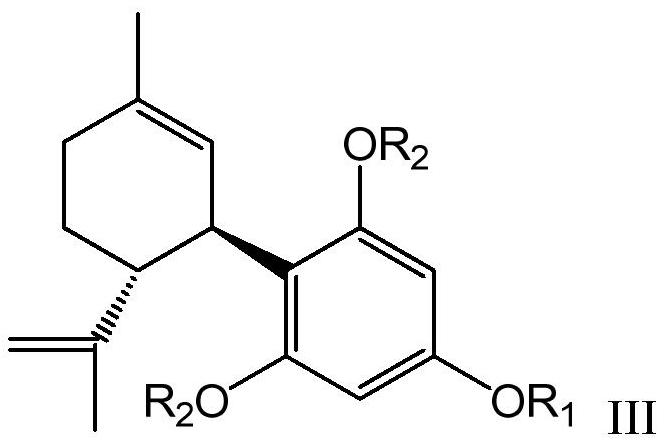
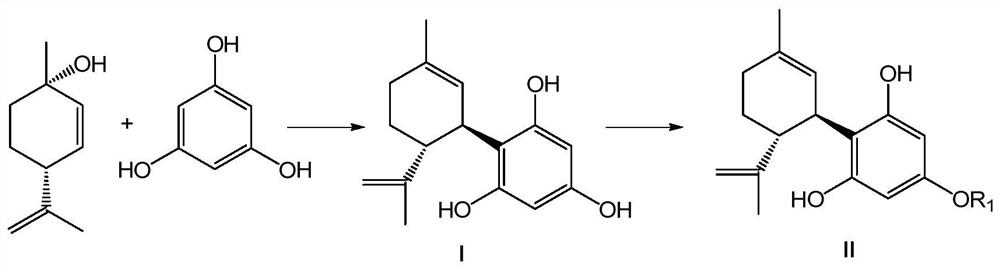
Resorcinol derivative and preparation method thereof
A compound and general formula technology, applied in the field of resorcinol derivatives and its preparation, can solve problems such as low product yield, low reaction regioselectivity, and difficult routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1: the preparation of compound I-1
[0098]
[0099] Phloroglucinol (50.4 g, 400 mmol) was added to a reaction flask with a constant pressure funnel. The air was removed under vacuum and replaced with nitrogen three times, the solvent tetrahydrofuran (150 mL) was added and stirred well until the solid dissolved and became clear. Then boron trifluoride-diethyl ether (2.85 g, 20 mmol) was added to the solution, and after stirring evenly, the reaction temperature was lowered to -5~5°C. Then a solution of trans-menthyl-2,8-dien-1-ol (4.04g, 40mmol) in tetrahydrofuran (50mL) was slowly added dropwise from a constant pressure funnel to the mixture under stirring at -5~5°C, Keep the reaction temperature at -5°C to 5°C for 4 hours. Saturated aqueous sodium bicarbonate (20 mL) was added to quench the reaction, and the reaction mixture was warmed to 20°C-25°C and stirred for half an hour. The aqueous phase was separated and discarded, the organic phase was distil...
Embodiment 2
[0101] Embodiment 2: the preparation of compound II-1
[0102]
[0103] Add compound I-1 (37.2g, 143mmol) and dichloromethane (80mL) into a reaction flask with a constant pressure funnel, add 2,6-lutidine (16.7g, 156mmol) and stir at room temperature . Add trifluoromethanesulfonic anhydride (36.6 g, 130 mmol) and dichloromethane (80 mL) into a constant pressure funnel and mix well. Slowly add the trifluoromethanesulfonic anhydride solution to the reaction under stirring at -20~-15°C, and the dropwise addition is completed in about 1 hour, then the reaction temperature is raised to 0~5°C and the reaction is continued for 3~4 hours. The reaction mixture was warmed up to room temperature 20-25°C, and dilute hydrochloric acid (1M, 35 mL) was added with stirring to quench the reaction and adjust the pH value to 1-2. After fully stirring, let it stand for liquid separation, separate the liquid and discard the aqueous phase. The organic phase was washed once with water, then to...
Embodiment 3
[0105] Embodiment 3: the preparation of compound III-1
[0106]
[0107] Add compound II-1 (3.92g, 10mmol) and dichloromethane (10mL) into a reaction flask with a constant pressure funnel at room temperature and stir until it dissolves and becomes clear, then add N,N-dimethylaminopyridine (122mg, 1mmol) and triethylamine (3.03g, 30mmol) and stirred evenly. Add pivaloyl chloride (3.01 g, 25 mmol) and dichloromethane (80 mL) into a constant pressure funnel and mix well. The pivaloyl chloride solution was slowly added dropwise to the reaction with stirring at room temperature, and the dropwise addition was completed in about 1 hour, and then the reaction was continued at room temperature for 10 to 12 hours. Dilute hydrochloric acid (1M, 15mL) was added under stirring to quench the reaction and adjust the pH to 1-2. After fully stirring, let it stand for liquid separation, separate the liquid and discard the aqueous phase. Saturated aqueous sodium bicarbonate (20 mL) was add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com