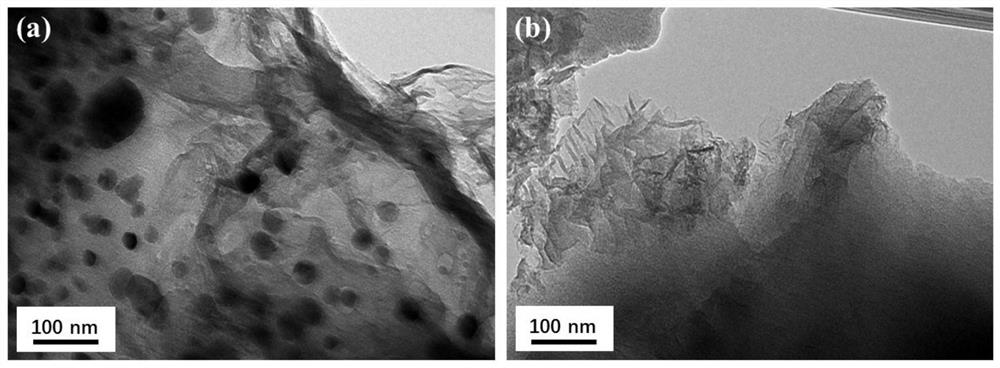
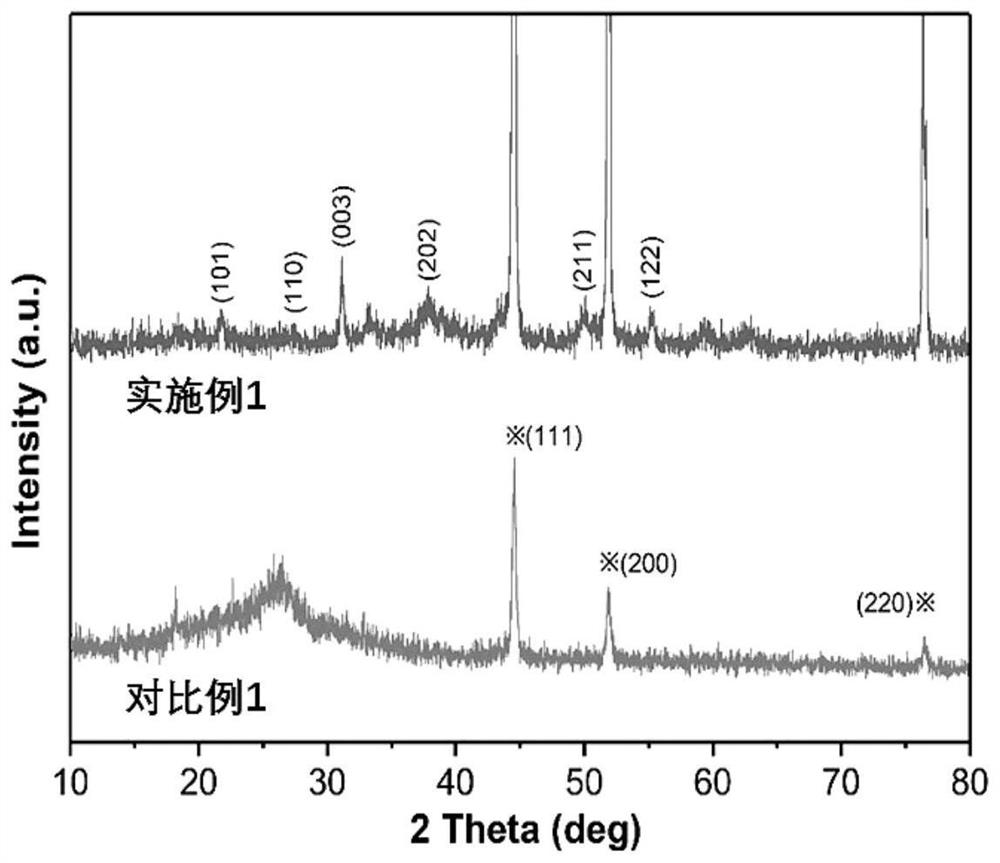
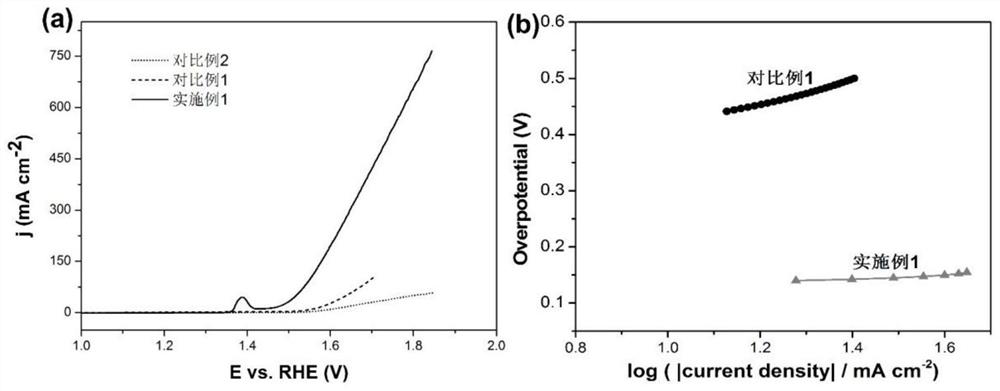
Ni/Ni3S2 nanocluster-graphene composite material as well as preparation method and application thereof
A composite material and graphene technology, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve problems such as hindering potential catalytic performance, low electrical conductivity, poor electrochemical stability, etc., to reduce Electron transfer barrier, enhanced conductivity, and optimized catalytic activity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The present embodiment 1 provides a kind of Ni / Ni supported by graphene 3 S 2 The preparation method of nano-cluster composite material, it comprises the steps:
[0053] (1) Inoculate the Pandoraea sp.B-6 cells stored on the LB slant in the LB liquid medium, and cultivate at 30°C for 18 hours to obtain the seed liquid of Pandoraea sp.B-6; wherein the LB liquid The distribution ratio of each component of the medium is: 10g of peptone, 5g of yeast powder, 10g of sodium chloride, and 1L of distilled water; the LB slope is based on the above formula by adding 15g / L of agar;
[0054] (2) The obtained Pandoraea sp.B-6 seed liquid was centrifuged for 5 minutes under the condition of 8000rpm, the supernatant was discarded, and the thalline was collected;
[0055] (3) Inoculate the collected Pandoraea sp.B-6 bacteria into 10% inoculum amount (the ratio of the volume of the transferred bacterial solution to the volume of the culture solution after inoculation), inoculate the st...
Embodiment 2
[0065] (1) Cultivate according to steps (1) and (2) in Example 1 to obtain the seed liquid of Pandoraea sp.B-6.
[0066] (2) The obtained Pandoraea sp.B-6 seed liquid was centrifuged for 5 minutes under the condition of 8000rpm, the supernatant was discarded, and the thalline was collected;
[0067] (3) Inoculate the collected Pandoraea sp.B-6 bacteria into 10% inoculum amount (the ratio of the volume of the transferred bacterial solution to the volume of the culture solution after inoculation), inoculate the sterile medium containing Cd, and inoculate at 30°C , natural pH, cultivated for 18h, and centrifuged at 8,000rpm for 5min to separate the bacterial cells; wherein the composition ratio of the Cd-containing sterile medium is: glucose 2g / L, Cd(NO 3 ) 2 , 0.3mM, NH 4 Cl1.5 g / L, MgCl 2 0.2g / L, CaCl 2 0.01g / L, FeSO 4 ·7H 2 O 0.015g / L, MnSO 4 ·H 2 O 0.01g / L; MOPs8.314g / L, trineg / L, L-cysteine 0.1mM.
[0068] (4) Disperse the CdS-bacteria precursor obtained in the ...
Embodiment 3
[0074] (1) Cultivate according to steps (1) and (2) in Example 1 to obtain the seed liquid of Pandoraea sp.B-6.
[0075] (2) The obtained Pandoraea sp.B-6 seed liquid was centrifuged for 5 minutes under the condition of 8000rpm, the supernatant was discarded, and the thalline was collected;
[0076] (3) Inoculate the collected Pandoraea sp.B-6 bacteria into 10% inoculum amount (the ratio of the volume of the transferred bacterial solution to the volume of the culture solution after inoculation), inoculate the sterile medium containing Cd, and inoculate at 30°C , natural pH, cultivated for 18h, and centrifuged at 8,000rpm for 5min to separate the bacterial cells; wherein the composition ratio of the Cd-containing sterile medium is: glucose 2g / L, Cd(NO 3 ) 2 , 0.4mM, NH 4 Cl1.5 g / L, MgCl 2 0.2g / L, CaCl 2 0.01g / L, FeSO 4 ·7H 2 O 0.015g / L, MnSO 4 ·H 2 O 0.01g / L; MOPs8.314g / L, trineg / L, L-cysteine 0.1mM.
[0077] (4) Disperse the CdS-bacteria precursor obtained in the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com