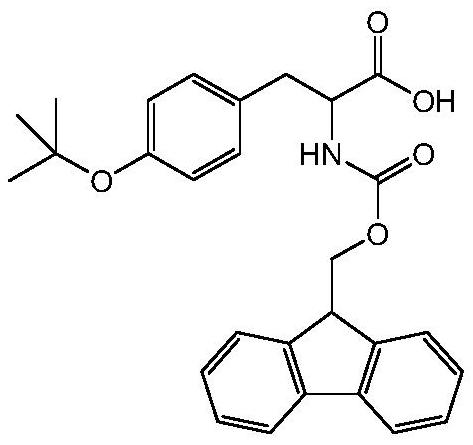
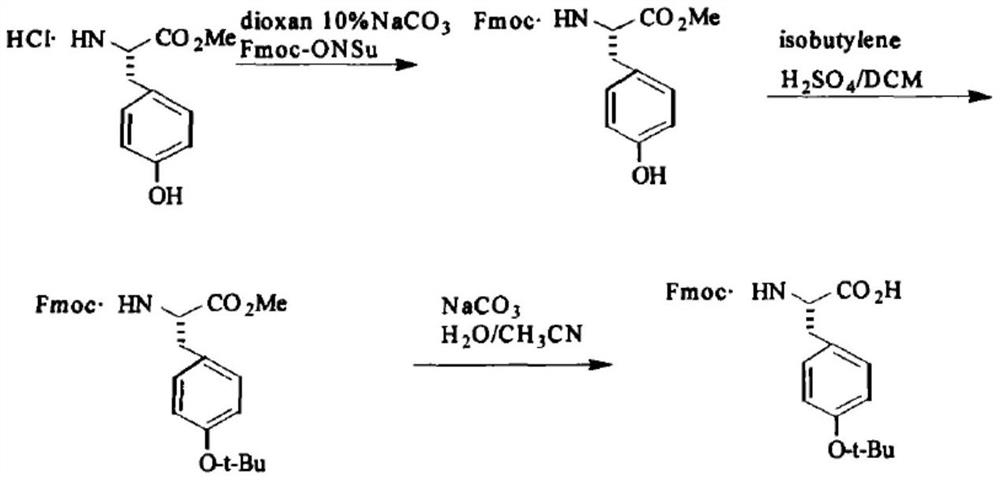
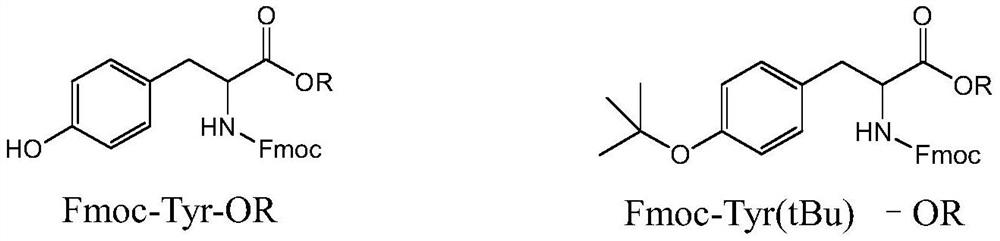
Method for preparing Fmoc-Tyr (tBu)-OH
A fmoc-tyr-or, C1-C4 technology, applied in the field of preparing Fmoc-Tyr-OH, can solve the problems of easy formation of explosive mixture, potential safety hazards, poor safety, etc., and achieve shortened production steps, low cost, and improved production efficiency. and the effect of yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Weigh and add 18 grams of L-Tyr (tyrosine) and 100 ml of methanol into the reaction bottle, and add SOCl dropwise under stirring. 2 (thionyl chloride) 120ml, reflux reaction. When TLC (thin-layer chromatography) detects that there is no L-Tyr (tyrosine) in the reaction system, the reaction is complete. The reaction solution that has completed the above reaction is vacuum concentrated to a volume of 20 ml, and 100 ml of petroleum ether is added to separate out a white solid. After centrifugation and drying, 19.2 g of white solid was obtained, with a yield of 95.3%.
[0054] 19.2 g of Tyr-OCH will be obtained 3 Add HCl to 160ml acetone and 40ml water, adjust pH=8-9 with sodium carbonate, add 33.7 g Fmoc-OSu, react at room temperature for 8 hours, adjust pH=2-3 with 1M dilute hydrochloric acid, and precipitate white solid Fmoc-Tyr- OCH 3 , filtered, and dried to obtain 39.8 grams of white solid, with a yield of 95.4%.
[0055] The resulting 39.8 g of Fmoc-Tyr-OCH 3 , ...
Embodiment 2
[0058] Weigh and add 18g of L-Tyr (tyrosine) and 100ml of ethanol to the reaction flask, and add SOCl dropwise under stirring. 2 (thionyl chloride) 120ml, reflux reaction. When TLC (thin-layer chromatography) detects that there is no L-Tyr (tyrosine) in the reaction system, the reaction is complete. The reaction solution that has completed the above reaction is vacuum concentrated to a volume of 20 ml, and 100 ml of petroleum ether is added to separate out a white solid. After centrifugation and drying, 19.8 g of white solid was obtained, with a yield of 94.7%.
[0059] Add the obtained 19.8 grams of Tyr-OEt·HCl to 160 ml of acetone and 40 ml of water, adjust the pH to 8-9 with sodium carbonate, add 33.7 grams of Fmoc-OSu, react at room temperature for 8 hours, and adjust the pH to 2-3 with 1M dilute hydrochloric acid , precipitated white solid Fmoc-Tyr-OEt, filtered and dried to obtain 40.1 g of white solid with a yield of 93.2%.
[0060] The obtained 40.1 g of Fmoc-Tyr-OEt...
Embodiment 3
[0063] Weigh and add 180g of L-Tyr (tyrosine) and 1000ml of methanol into the reaction flask, add SOCl dropwise under stirring 2 (Thionyl chloride) 1200ml, reflux reaction. When TLC (thin-layer chromatography) detects that there is no L-Tyr (tyrosine) in the reaction system, the reaction is complete. The reaction solution that has completed the above reaction is vacuum concentrated to a volume of 20 ml, and 100 ml of petroleum ether is added to separate out a white solid. Centrifuge and dry to obtain 191 g of white solid, yield 95.0%.
[0064] 191 grams of Tyr-OCH will be obtained 3 Add 1600ml acetone and 400ml water with HCl, adjust pH=8-9 with sodium carbonate, add 337g Fmoc-OSu, react at room temperature for 8 hours, adjust pH=2-3 with 1M dilute hydrochloric acid, precipitate white solid Fmoc-Tyr- OCH 3 , filtered, and dried to obtain 398 grams of white solid, with a yield of 95.4%.
[0065] The resulting 398 g of Fmoc-Tyr-OCH 3 , join in 5000ml tert-butyl acetate and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com