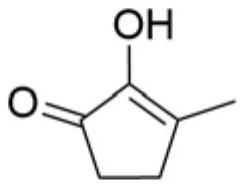
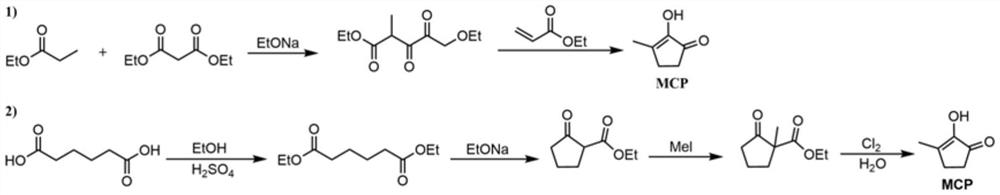
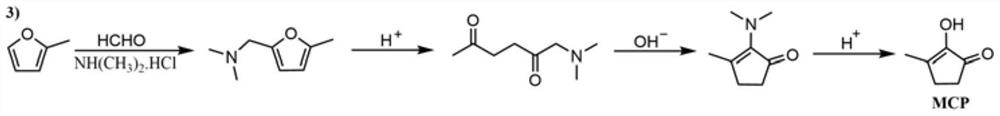
Synthetic method of methyl cyclopentenolone
A technology of methyl cyclopentenol ketone and synthesis method, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc. higher question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] A synthetic method of methylcyclopentenolone, the method may further comprise the steps:
[0031] (1) Control reaction system at pH=2-7, preferably pH=3-6, temperature is 45-70 DEG C, under preferred 50-65 DEG C, monitor dimethylamine hydrochloride, formaldehyde aqueous solution and 2 with online infrared instrument -Methylfuran reaction generates N,N-dimethyl-5-methylfurfurylamine reaction process; wherein, the mol ratio of dimethylamine hydrochloride and formaldehyde is (1-1.2):1, preferably (1- 1.02):1; the mass concentration of formaldehyde aqueous solution is 37-40ωt%; the molar ratio of dimethylamine hydrochloride to 2-methylfuran is (1-1.3):1. Preferred (1.05-1.07):1.
[0032] When detecting the content of 2-methylfuran in the reaction solution<0.55%, then acidify the reaction system pH<1 with industrial hydrochloric acid with a concentration of 5-37ωt%, preferably 30ωt%, at 60-100°C, preferably 70-100°C, The time is 1-4h, preferably 2-3h to obtain the product ...
Embodiment 1
[0036] A synthetic method of methylcyclopentenolone, the method may further comprise the steps:
[0037] (1) Add 44.85g (0.55mol) dimethylamine hydrochloride and 19.59g (1.09mol) water into a 500ml four-neck flask, control the temperature to 50°C, add 42.08g (37-40%, 0.54mol) formaldehyde , adjust the pH of the reaction solution to 4.5, and start to add 42.69g (0.52mol) of 2-methylfuran dropwise after 0.5h of reaction;
[0038] Leverage React IR TM 15 Monitor the reaction solution and find that the content of 2-methylfuran is <0.55%, while the mass content of N,N-dimethyl-5-methylfurfurylamine is 46.24%. Then dilute hydrochloric acid is added to adjust the pH to 0.95, and the temperature is raised to 70 ℃, after 3 hours of reaction, adjust the pH to 12.5 with sodium hydroxide solution, and react at a temperature of 75-80℃ for 0.5 hours to obtain 2-(dimethylamino)-3-methyl-2-cyclopentene-1- The ketone content is 95.57%. After extraction, concentration and recovery of the sol...
Embodiment 2
[0042] A synthetic method of methylcyclopentenolone, the method may further comprise the steps:
[0043] (1) Add 44.85g (0.55mol) dimethylamine hydrochloride and 19.59g (1.09mol) water into a 500ml four-neck flask, control the temperature to 60°C, add 42.08g (37-40%, 0.54mol) formaldehyde , adjust the pH of the reaction solution to 4.5, and start to add 42.69g (0.52mol) of 2-methylfuran dropwise after 0.5h of reaction,
[0044] Leverage React IR TM 15 Monitor the reaction solution and find that the content of 2-methylfuran is <0.55%, while the content of N,N-dimethyl-5-methylfurfurylamine is 48.27%, then add dilute hydrochloric acid, adjust the pH to 0.95, and heat up to 80°C , after reacting for 3 hours, adjust the pH to 12.5 with sodium hydroxide solution, and react at a temperature of 75-80°C for 0.5 hours to obtain 2-(dimethylamino)-3-methyl-2-cyclopenten-1-one The content is 95.61%. After extraction, concentration and recovery of the solvent, 2.21g of the front fractio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com