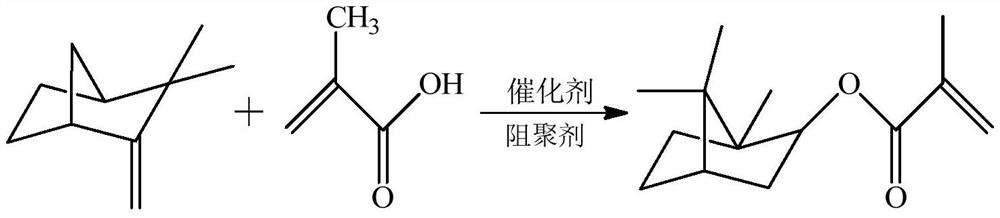
A kind of preparation method of isobornyl acrylate of biological origin
A technology of isobornyl acrylate and methacrylic acid, which is applied in the field of photocatalytic organic synthesis, and can solve problems such as uneven heating, low efficiency, and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 136g camphene (1.0mol) in 129g methacrylic acid (1.5mol), dissolve, stir well, add 26.5g (10%) phosphotungstic acid / silica gel catalyst (HPW / SiO 2 ) and 0.129g (0.1%) inhibitor hydroquinone, stir evenly, place light under a 100W UV light source, react while stirring at a speed of 100r / min, stop after reacting for 10min, and obtain The pure and transparent isobornyl methacrylate has a measured yield of 91.2%.
[0031] The molecular weight of the synthesized product was measured with an Agilent 7250GC / Q-TOF GC / MS system from Agilent Corporation of the United States, and the relative molecular mass of the product was measured to be 222, which was the same as the theoretical relative molecular mass of isobornyl methacrylate.
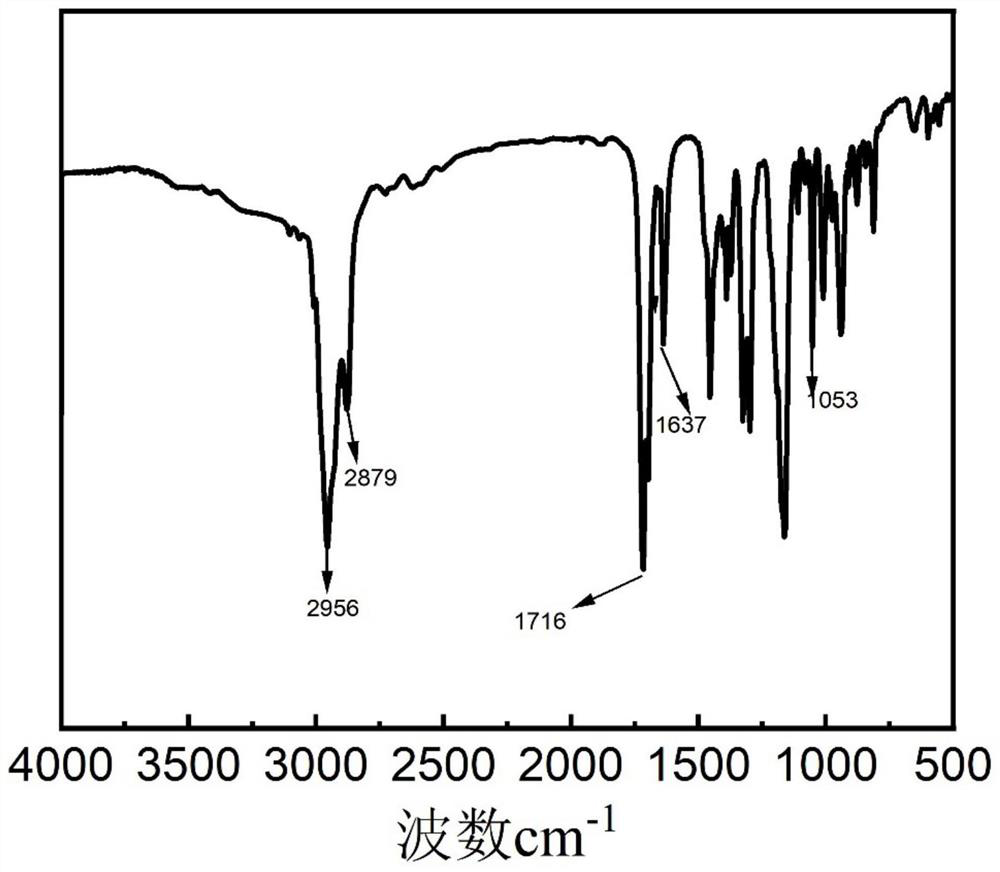
[0032] Test sample with American Nicolet company Thermo-Nicolet iS10 type Fourier transform infrared spectrometer, the infrared spectrum of the synthesized isobornyl methacrylate is as follows: figure 1 shown. 2880~2966cm -1 C-H stretching vibr...
Embodiment 2
[0034] Add 136g camphene (1.0mol) in 72g acrylic acid (1.0mol), dissolve, stir well, add 2.22g (1%) catalyst cationic photoinitiator triarylsulfonium salt (UVI 6976 of Dow Chemical Company) and 0.172 g (0.2%) polymerization inhibitor p-hydroxyanisole, stir evenly, place it under a 200W UV light source, react while stirring at a speed of 200r / min, stop after 20min, and obtain pure and transparent by rotary evaporation under reduced pressure The isobornyl acrylate is recorded as 92.4% in yield.
[0035] The molecular weight of the synthesized product was measured with an Agilent 7250GC / Q-TOF GC / MS system from Agilent Corporation of the United States, and the relative molecular mass of the product was measured to be 208, which was the same as the theoretical relative molecular mass of isobornyl acrylate.
[0036] The sample was tested with a Thermo-Nicolet iS10 Fourier transform infrared spectrometer from Nicolet Company of the United States, and the characteristic peaks on the t...
Embodiment 3
[0038]Add 136g camphene (1.0mol) in 43g methacrylic acid (0.5mol), dissolve, stir well, add 8.95g (5%) catalyst acidic ionic liquid N-(4-sulfonic acid group) butyl trimethylamine hydrogen sulfate Salt and 0.129g (0.3%) inhibitor phenothiazine, stir evenly, place light under the UV light source of 300W, react while stirring with the rotating speed of 300r / min, stop after reacting for 30min, decompression rotary evaporation promptly obtains pure The yield of transparent isobornyl methacrylate was measured to be 90.8%.
[0039] The molecular weight of the synthesized product was measured with an Agilent 7250GC / Q-TOF GC / MS system from Agilent Corporation of the United States, and the relative molecular mass of the product was measured to be 222, which was the same as the theoretical relative molecular mass of isobornyl methacrylate.
[0040] The sample was tested with a Thermo-Nicolet iS10 Fourier transform infrared spectrometer from Nicolet Company of the United States, and the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com