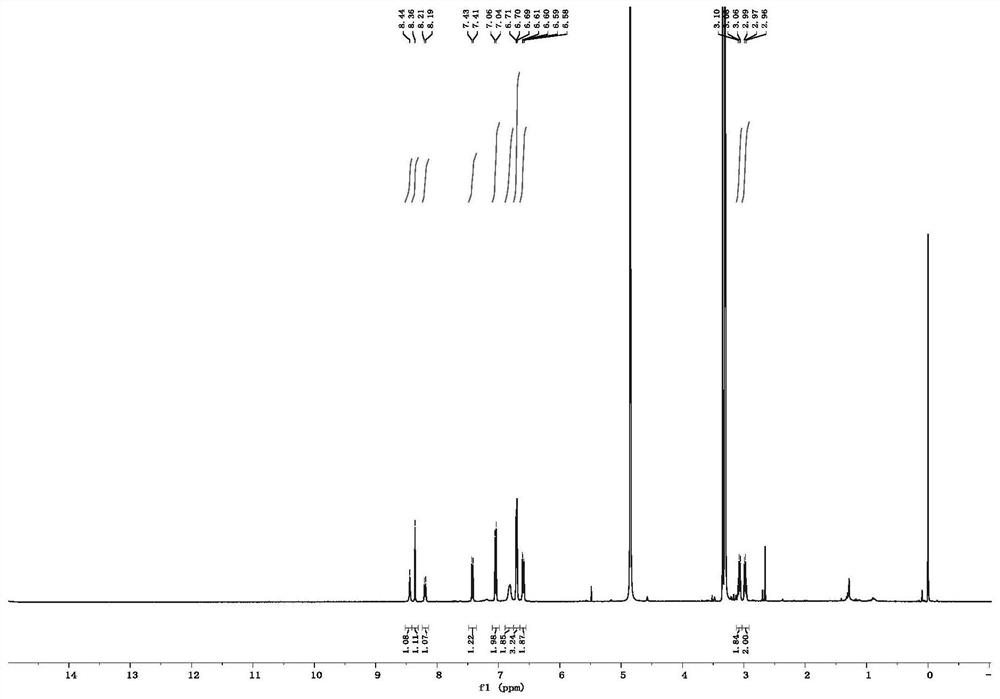
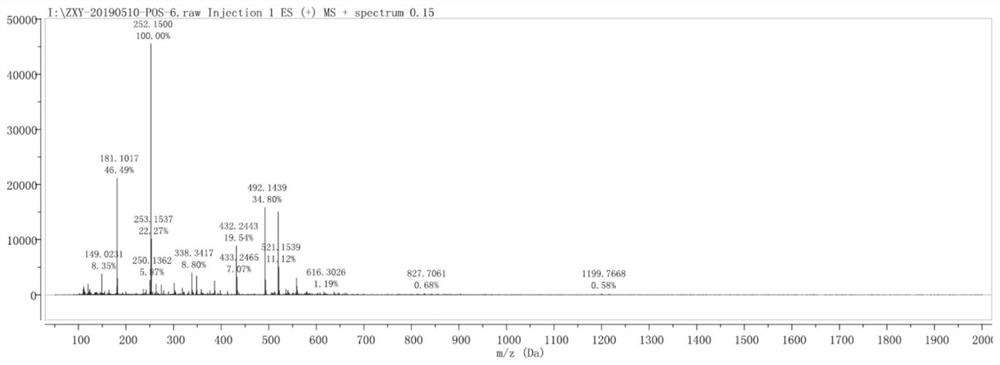
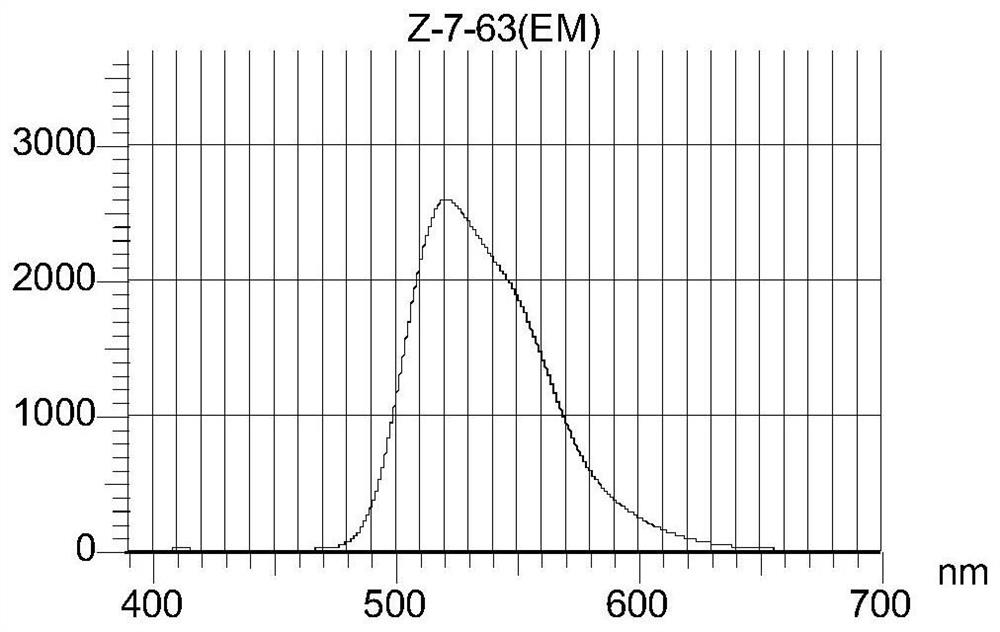
Preparation method of fluorescein isothiocyanate derivative
A technology of fluorescein isothiocyanate and derivatives, applied in organic chemistry and other directions, to achieve the effects of good luminescence effect, high product purity and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation method of compound 1
[0059]
[0060] Reactions were performed in dry glassware under an argon atmosphere. The starting 4-(but-3-yn-1-yl)phenol alcohol (669 mg, 2.4 mmol, 1.0 equiv) was dissolved in anhydrous dichloromethane (10 mL) and washed with Et3 SiH (773 μL, 563 mg, 4.8 mmol, 2.0 eq) treated. The mixture was cooled to 0 °C, then BF was added 3 ·OEt 2 (613 μL, 687 mg, 4.8 mmol, 2.0 equiv) and the reaction was stirred at 0° C. for 1 hour. When TLC showed that the starting material was completely converted, another 4 equivalents of BF 3 ·OEt 2 (1.2 mL, 1.37 g, 9.6 mmol, 4.0 equiv), the mixture was warmed to room temperature and stirred for 18 hours. TLC showed complete conversion of the intermediate to the final product, the mixture was diluted with dichloromethane (50 mL) and washed with saturated NaHCO 3 (30 mL) aqueous solution. The aqueous layer was back extracted with dichloromethane (2×20 mL), the combined organic layers were washed ...
Embodiment 2
[0062] The preparation method of compound 3
[0063]
[0064] Dissolve 1,4-benzenediol (20mmol), propargyl bromide (10mmol) and potassium carbonate (15mmol) in acetonitrile (50mL) and react overnight at 50°C. The solvent was removed by rotary evaporation, and the target product 3 was obtained by separation by column chromatography.
Embodiment 3
[0066] The preparation method of compound 5
[0067]
[0068] 5 mmol of the compound 5-aminofluorescein (purchased from Aladdin, Lot No. H1801120) was dissolved in 100 mL of acetic acid / water (2:1), and 7.5 mmol of sodium nitrite was added at 0°C. After 15 minutes of reaction, 10 mmol of sodium azide was added, and the reaction was continued for 2 hours. After the reaction, the solvent was removed by rotary evaporation, and the crude product was washed successively with 200 mL of 2M hydrochloric acid and 1000 mL of water. TLC detection reaction. Purification by column chromatography (dichloromethane:methanol=8:1) gave product 5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com