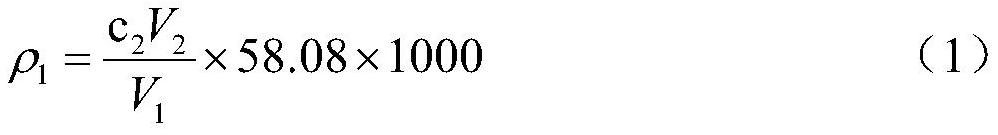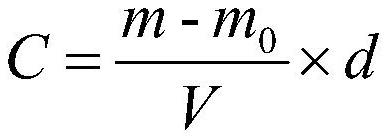Method for removing hypochlorite and ferric iron interference in thiocyanate measurement
A technology of thiocyanate and hypochlorite, which is used in the measurement of color/spectral characteristics, material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, etc., can solve the problem of thiocyanate Solve problems such as inaccurate determination of salt, achieve the effect of shortening the decomposition time and increasing the catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Take a 50mL sample and place it in a 250mL beaker, and add 1mL of Na to the sample 2 CO 3 solution;
[0052] (2) Add 30% hydrogen peroxide solution dropwise until the solution no longer produces bubbles, and an excess of 0.5 mL;
[0053] (3) Heat 0.4g of manganese dioxide powder and 0.5mL of copper chloride solution, cover with a watch glass, heat on an electric hob at a heating temperature of 150°C, heat for 20 minutes, remove the beaker, and cool slightly;
[0054] (4) Filter the solution into a 250mL beaker, wash the precipitate and the filter paper twice, add hydrochloric acid to adjust the pH value of the solution, and adjust it to neutral;
[0055] (5) After adjusting the pH value, the filtrate is settled in a 200mL volumetric flask;
[0056](6) measure the content of ferric iron in the volumetric flask in step (5), measure according to the mensuration o-phenanthroline spectrophotometry of standard HJ / T 345─2007 water quality iron, measure the content of to...
Embodiment 2
[0071] (1) Take a 50mL sample and put it in a 250mL beaker. The sample is strongly alkaline. Add hydrochloric acid to adjust the pH value to 8-8.5. Add 1mL Na 2 CO 3 solution;
[0072] (2) Add 30% hydrogen peroxide solution dropwise until the solution no longer produces bubbles, and an excess of 0.5 mL;
[0073] (3) Heat 0.4g of manganese dioxide powder and 0.5mL of copper chloride solution, cover with a watch glass, heat on an electric stove, the heating temperature is between 160°C, heat for 20 minutes, remove the beaker, and cool slightly;
[0074] (4) Filter the solution into a 250mL beaker, wash the precipitate and the filter paper twice, add hydrochloric acid to adjust the pH value of the solution, and adjust it to neutral;
[0075] (5) After adjusting the pH value, the filtrate is settled in a 200mL volumetric flask;
[0076] (6) measure the content of ferric iron in the volumetric flask in step (5), measure according to standard HJ / T 345─2007 water quality, the mens...
Embodiment 3
[0091] Proceed as follows:
[0092] (1) Take a 50mL sample and place it in a 250mL beaker. If the sample is weakly acidic, add 1mL Na 2 CO 3 solution;
[0093] (2) Add 30% hydrogen peroxide solution dropwise until the solution no longer produces bubbles, and an excess of 0.5 mL;
[0094] (3) Heat 0.4g of manganese dioxide powder and 0.5mL of copper chloride solution, cover with a watch glass, heat on an electric hob at a heating temperature of 170°C, heat for 20 minutes, remove the beaker, and cool slightly;
[0095] (4) Filter the solution into a 250mL beaker, wash the precipitate and the filter paper twice, add hydrochloric acid to adjust the pH value of the solution, and adjust it to neutral;
[0096] (5) After adjusting the pH value, the filtrate is settled in a 200mL volumetric flask;
[0097] (6) measure the content of ferric iron in the volumetric flask in step (5), measure according to the mensuration o-phenanthroline spectrophotometry of standard HJ / T 345─2007 wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com