Fluorination method
A technology of fluorination and fluorination reagents, applied in organic chemistry methods, chemical instruments and methods, organic chemistry and other directions, can solve the problems of low stability and high cost, and achieve the effect of good yield and high tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The fluorinated reagent can be prepared by existing preparation methods:
[0048] For example, in some embodiments, the perfluoropolyether chain carboxylate provided by the present invention can be prepared by reacting a perfluoropolyether chain carboxylate with a base.
[0049] In some embodiments, the molar ratio of the substrate to the fluorinating reagent is 1:0.25-2.
[0050] In some embodiments, the fluorination reaction is performed in an organic solvent system.
[0051] In some embodiments, the reaction temperature of the fluorination reaction is 50°C-150°C.
[0052] In some embodiments, when the substrate is selected from carboxylic acid compounds, the fluorination reaction is carried out in an organic solvent, the reaction temperature is 50°C-135°C, and the molar ratio of the substrate to the fluorinating reagent is 1:0.5- 2.
[0053] In a preferred embodiment, when the substrate is selected from carboxylic acid compounds, the organic solvent is selected fr...
Embodiment 1
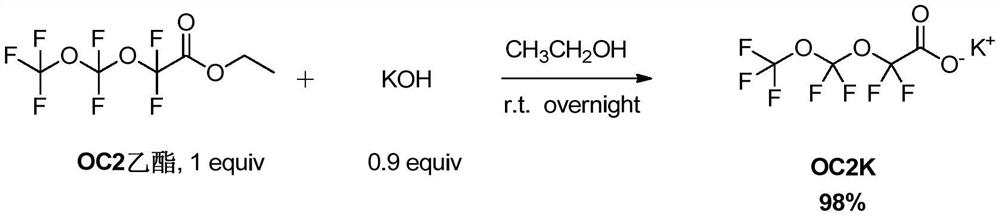
[0076] In this example, CF 3 OCF 2 OCF 2 CO 2 K is an example to illustrate the preparation method of perfluoropolyether chain carboxylate provided by the present invention, comprising the following operations:
[0077] Add CF to a 500mL single-necked bottle 3 OCF 2 OCF 2 CO 2 C 2 h 5 (54.8g, 0.2mol) and 150mL of ethanol, and KOH (10.1g, 0.18mol) was dissolved in 10mL of water, and the KOH aqueous solution was added dropwise into the single-necked bottle with a syringe, and stirred vigorously after the addition, and reacted overnight at room temperature . After the reaction was completed, the solvent was removed under reduced pressure, and the water was removed overnight with an oil pump under the condition of heating at 50 °C to obtain 55.7 g of white solid CF 3 OCF 2 OCF 2 CO 2 K.
[0078] Concrete reaction formula is as follows:
[0079]
[0080] The present invention will be further illustrated by the fluorination reaction examples of carboxylic acid comp...
Embodiment 2
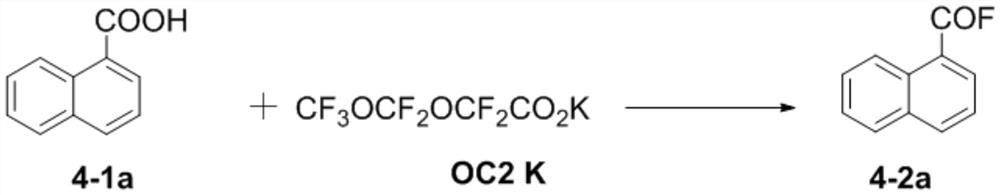
[0082] This embodiment is used to illustrate the fluorination method disclosed by the present invention, comprising the following steps:
[0083] Weigh carboxylic acid substrate 4-1a (0.2mmol, 1.0equiv) and fluorinated reagent CF in a 10mL dried Shrek tube 3 OCF 2 OCF 2 CO 2 K (0.2mmol, 1.0equiv), replace the nitrogen three times, add anhydrous acetonitrile 1mL under the protection of nitrogen, the reaction temperature is 80°C, and react for 1h. Cool to room temperature after the reaction to obtain the reaction product, filter and purify the reaction product through a silica gel plug, wash the silica gel plug with a mixture of petroleum ether / ethyl acetate (150ml; 20:1~5:1, v:v), and spin the filtrate to dry the solvent That is, the final product 4-2a was obtained.
[0084] Concrete reaction formula is as follows:
[0085]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com